Artemisin
 From Handwiki
From Handwiki Short description: Chemical compound
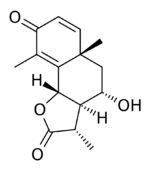
| |
| Names | |
|---|---|
| Preferred IUPAC name
(3S,3aR,4S,5aS,9bS)-4-Hydroxy-3,5a,9-trimethyl-3a,5,5a,9b-tetrahydronaphtho[1,2-b]furan-2,8(3H,4H)-dione | |
| Identifiers | |
CAS Number
|
|
3D model (JSmol)
|
|
| ChEBI |
|
| ChEMBL |
|
| ChemSpider |
|
| KEGG |
|
PubChem CID
|
|
| UNII |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula
|
C15H18O4 |
| Molar mass | 262.305 g·mol−1 |
| Melting point | 203 °C (397 °F; 476 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
- SizeSet
Artemisin is a sesquiterpene lactone, similar in structure to α-santonin.[1][2]
See also
- Artemisia (genus), hardy herbaceous plants and shrubs known for the powerful chemical constituents in their essential oils
- Artemisinin, a group of drugs used against malaria
- Santonin, an anthelminthic, drug expelling parasitic worms (helminths) by paralyzing them
References
- ↑ SUMI, Masao (1956). "The Structure of Artemisin". Proceedings of the Japan Academy 32 (9): 684–687. doi:10.2183/pjab1945.32.684.
- ↑ ApSimon, John (2009) (in en). The Total Synthesis of Natural Products. John Wiley & Sons. ISBN 9780470129517. https://books.google.com/books?id=MhEDN-d1Dw8C&pg=PA324.
External links
 |
Categories: [Sesquiterpene lactones]
↧ Download as ZWI file | Last modified: 05/25/2024 20:27:28 | 2 views
☰ Source: https://handwiki.org/wiki/Chemistry:Artemisin | License: CC BY-SA 3.0
✘
ZWI is not signed. [what is this?]

 KSF
KSF