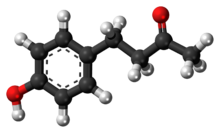
Raspberry ketone
Topic: Chemistry
 From HandWiki - Reading time: 4 min
From HandWiki - Reading time: 4 min

| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
4-(4-Hydroxyphenyl)butan-2-one | |
| Other names
p-Hydroxybenzyl acetone; 4-(p-Hydroxyphenyl)-2-butanone; Frambinone; Oxyphenylon; Rheosmin; Rasketone
| |
| Identifiers | |
3D model (JSmol)
|
|
| Abbreviations | RK |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C10H12O2 | |
| Molar mass | 164.204 g·mol−1 |
| Appearance | White needles[2] |
| Melting point | 82 to 84 °C (180 to 183 °F; 355 to 357 K) |
| Boiling point | 140 to 146 °C (284 to 295 °F; 413 to 419 K) at 0.5 mmHg |
| Hazards | |
| GHS pictograms | 
|
| GHS Signal word | Warning |
| H302 | |
| P264, P270, P301+312, P330, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Raspberry ketone is a natural phenolic compound that is the primary aroma compound of red raspberries.
Occurrence
Raspberry ketone occurs in a variety of fruits, including raspberries, cranberries, and blackberries.[3] It is detected and released by orchid flowers, e.g. Dendrobium superbum (syn D. anosmum),[4] and several Bulbophyllum species[5][6][7] to attract raspberry ketone-responsive male Dacini fruit flies. It is biosynthesized from coumaroyl-CoA.[8] It can be extracted from the fruit, yielding about 1–4 mg per kg of raspberries.[9]
Preparation
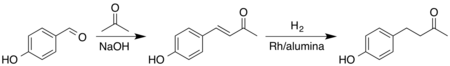
Since the natural abundance of raspberry ketone is very low, it is prepared industrially by a variety of methods from chemical intermediates.[10] One of the ways this can be done is through a Claisen-Schmidt condensation followed by catalytic hydrogenation. First, acetone is condensed with 4-hydroxybenzaldehyde to form an α,β-unsaturated ketone. Then the alkene part is reduced to the alkane. This two-step method produces raspberry ketone in 99% yield.[11] There is a less expensive hydrogenation catalyst, nickel boride, which also demonstrates high selectivity towards hydrogenation of the double bond of enone.[12]
Uses
Raspberry ketone is sometimes used in perfumery, in cosmetics, and as a food additive to impart a fruity odor. It is one of the most expensive natural flavor components used in the food industry. The natural compound can cost as much as $20,000 per kg.[9]
Marketing
Although products containing this compound are marketed for weight loss, there is no clinical evidence for this effect in humans.[13][14] They are called "ketones" because of the ketone (acetone) group at their end, which is shared with ketone bodies.
Safety
Little is known about the long-term safety of raspberry ketone supplements,[15][16] especially since little research has been done with humans.[17] Toxicological models indicate a potential for cardiotoxic effects, as well as effects on reproduction and development.[15] Furthermore, in many dietary supplements containing raspberry ketones, manufacturers add other ingredients such as caffeine which may have unsafe effects.[17]
In 1965, the US Food and Drug Administration classified raspberry ketone as generally recognized as safe (GRAS) for the small quantities used to flavor foods.[2]
See also
References
- ↑ Catalog of Organics and Fine Chemicals, Acros Organics, 2004/05, page 1250.
- ↑ 2.0 2.1 "4-(p-Hydroxyphenyl)-2-butanone". Food and Cosmetics Toxicology 16: 781–2. 1978. doi:10.1016/S0015-6264(78)80113-8.
- ↑ "Raspberry Ketone, Molecule of the Month". University of Bristol. http://www.chm.bris.ac.uk/motm/raspberry-ketone/rkh.htm.
- ↑ Nishida, R.; Iwahashi, I.; Tan, K.H. (1993). "Accumulation of Dendrobium (Orchidaceae) flower fragrance in the rectal glands by males of the melon fly, Dacus cucurbitae (Tephritidae)". Journal of Chemical Ecology 19: 713–722. doi:10.1007/BF00985003.
- ↑ Tan, K.H.; Nishida, R. (2005). "Synomone or Kairomone? - Bulbophyllum apertum (Orchidaceae) flower releases raspberry ketone to attract Bactrocera fruit flies". Journal of Chemical Ecology 31 (3): 509–519. doi:10.1007/s10886-005-2023-8.
- ↑ Tan, K.H.; Tan, L.T. (2018). "Movements of floral parts and roles of the tooth on column wall of Bulbophyllum praetervisum (Orchidaceae) flower for pollination by Dacini fruit flies (Diptera: Tephritidae)". Journal of Pollination Ecology 24 (17): 157–163. doi:10.26786/1920-7603(2018)19.
- ↑ Nakahira, M.; Ono, H.; Wee, S.L.; Nishida, R. (2018). "Floral synomone diversification of Bulbophyllum sibling species (Orchidaceae) in attracting fruit fly pollinators". Biochemical Systematics and Ecology 81: 86–95. doi:10.1016/j.bse.2018.10.002.
- ↑ "MetaCyc Pathway: raspberry ketone biosynthesis". MetaCyc. http://biocyc.org/META/new-image?type=PATHWAY&object=PWY-5393. Retrieved 2012-07-12.
- ↑ 9.0 9.1 Beekwilder, Jules; Van Der Meer, Ingrid M.; Sibbesen, Ole; Broekgaarden, Mans; Qvist, Ingmar; Mikkelsen, Joern D.; Hall, Robert D. (2007). "Microbial production of natural raspberry ketone". Biotechnology Journal 2 (10): 1270–9. doi:10.1002/biot.200700076. PMID 17722151.
- ↑ Tateiwa, Jun-Ichi; Horiuchi, Hiroki; Hashimoto, Keiji; Yamauchi, Takayoshi; Uemura, Sakae (1994). "Cation-Exchanged Montmorillonite-Catalyzed Facile Friedel-Crafts Alkylation of Hydroxy and Methoxy Aromatics with 4-Hydroxybutan-2-one to Produce Raspberry Ketone and Some Pharmaceutically Active Compounds". The Journal of Organic Chemistry 59 (20): 5901–4. doi:10.1021/jo00099a017.
- ↑ Smith, Leverett R. (1996). "Rheosmin ('Raspberry Ketone') and Zingerone, and Their Preparation by Crossed Aldol-Catalytic Hydrogenation Sequences". The Chemical Educator 1 (3): 1–18. doi:10.1007/s00897960034a.
- ↑ Bandarenko, Mikhail; Kovalenko, Vitaly (2014). "Synthesis of Raspberry and Ginger Ketones by Nickel Boride-catalyzed Hydrogenation of 4-Arylbut-3-en-2-ones". Zeitschrift für Naturforschung B 69b (8): 885–888. doi:10.5560/ZNB.2014-4118.
- ↑ "Raspberry Ketones: Uses, Health Benefits, and Risks". WebMD. https://www.webmd.com/diet/raspberry-ketones-uses-risks.
- ↑ "Raspberry Ketone". WebMD. https://www.webmd.com/vitamins/ai/ingredientmono-1262/raspberry-ketone.
- ↑ 15.0 15.1 "Raspberry ketone in food supplements - High intake, few toxicity data - A cause for safety concern?". Regul Toxicol Pharmacol 73 (1): 196–200. 2015. doi:10.1016/j.yrtph.2015.06.022. PMID 26160596.
- ↑ Cathy Wong. "Raspberry Ketones for Weight Loss". About.com. http://altmedicine.about.com/od/weight_Loss/a/Raspberry-Ketones-Weight-Loss.htm.
- ↑ 17.0 17.1 Canberra, Jules. "What's All The Hype About Raspberry Ketone?". https://www.authorityhealth.com/raspberry-ketone-benefits. Retrieved 30 October 2017.
 |
 KSF
KSF