Loxapine (inhalation)
 From Wikidoc - Reading time: 21 min
From Wikidoc - Reading time: 21 min
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Kiran Singh, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
Warning:
See full prescribing information for complete Boxed Warning.
Bronchospasm
|
Overview
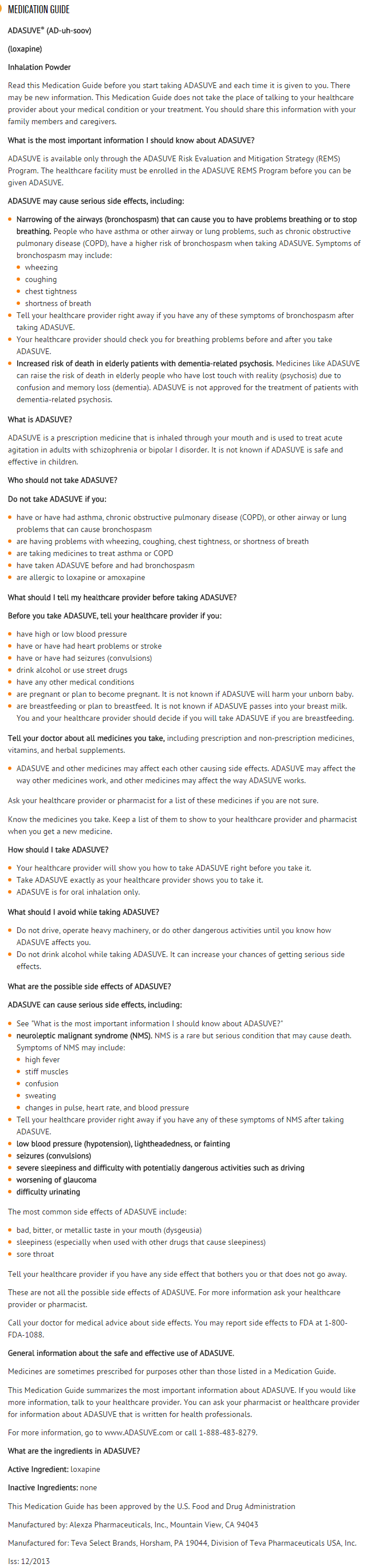
Loxapine (inhalation) is an antipsychotic drug that is FDA approved for the treatment of agitation associated with schizophrenia or bipolar I disorder in adults.. There is a Black Box Warning for this drug as shown here. Common adverse reactions include dysgeusia, sedation, and throat irritation.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indications
- ADASUVE is a typical antipsychotic indicated for the acute treatment of agitation associated with schizophrenia or bipolar I disorder in adults.
- "Psychomotor agitation" is defined in DSM-IV as "excessive motor activity associated with a feeling of inner tension." Patients experiencing agitation often manifest behaviors that interfere with their care (e.g., threatening behaviors, escalating or urgently distressing behavior, self-exhausting behavior), leading clinicians to the use of rapidly absorbed antipsychotic medications to achieve immediate control of the agitation.
- The efficacy of ADASUVE was established in one study of acute agitation in patients with schizophrenia and one study of acute agitation in patients with bipolar I disorder.
Limitations of Use:
- As part of the ADASUVE REMS Program to mitigate the risk of bronchospasm, ADASUVE must be administered only in an enrolled healthcare facility.
Dosing
- ADASUVE must be administered only by a healthcare professional. ADASUVE is administered by oral inhalation only. The recommended dose for acute agitation is 10 mg administered by oral inhalation, using a single-use inhaler. Administer only a single dose within a 24-hour period .
Required Examination Prior to Dosing
Prior to administering ADASUVE, screen all patients for a history of asthma, COPD, or other pulmonary disease, and examine patients (including chest auscultation) for respiratory signs (e.g. wheezing).
DOSAGE FORMS AND STRENGTHS
- ADASUVE is an inhalation powder supplied in a single-use, disposable inhaler containing 10 mg of loxapine base.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Loxapine (inhalation) in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Loxapine (inhalation) in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding FDA-Labeled Use of Loxapine (inhalation) in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Loxapine (inhalation) in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Loxapine (inhalation) in pediatric patients.
Contraindications
ADASUVE is contraindicated in patients with the following:
- Current diagnosis or history of asthma, COPD, or other lung disease associated with bronchospasm.
- Acute respiratory symptoms or signs (e.g., wheezing).
- History of bronchospasm following ADASUVE treatment
- Known hypersensitivity to loxapine or amoxapine. Serious skin reactions have occurred with oral loxapine and amoxapine.
Warnings
|
Warning:
See full prescribing information for complete Boxed Warning.
Bronchospasm
|
Bronchospasm
- ADASUVE can cause bronchospasm that has the potential to lead to respiratory distress and respiratory arrest . Administer ADASUVE only in an enrolled healthcare facility that has immediate access on-site to equipment and personnel trained to manage acute bronchospasm, including advanced airway management (intubation and mechanical ventilation).
- Prior to administering ADASUVE, screen patients regarding a current diagnosis or history of asthma, COPD, and other lung disease associated with bronchospasm, acute respiratory symptoms or signs, current use of medications to treat airways disease, such as asthma or COPD; and examine patients (including chest auscultation) for respiratory abnormalities (e.g., wheezing). Monitor patients for symptoms and signs of bronchospasm (i.e., vital signs and chest auscultation) at least every 15 minutes for a minimum of one hour following treatment with ADASUVE . ADASUVE can cause sedation, which can mask the symptoms of bronchospasm.
- Because clinical trials in patients with asthma or COPD demonstrated that the degree of bronchospasm, as indicated by changes in forced expiratory volume in 1 second (FEV1), was greater following a second dose of ADASUVE, limit ADASUVE use to a single dose within a 24 hour period.
- Advise all patients of the risk of bronchospasm. Advise them to inform the healthcare professional if they develop any breathing problems such as wheezing, shortness of breath, chest tightness, or cough following treatment with ADASUVE.
ADASUVE REMS to Mitigate Bronchospasm
- Because of the risk of bronchospasm, ADASUVE is available only through a restricted program under a REMS called the ADASUVE REMS.Required components of the ADASUVE REMS are:
- Healthcare facilities that dispense and administer ADASUVE must be enrolled and comply with the REMS requirements. Certified healthcare facilities must have on-site access to equipment and personnel trained to provide advance airway management, including intubation and mechanical ventilation.
- Wholesalers and distributors that distribute ADASUVE must enroll in the program and distribute only to enrolled healthcare facilities.
Increased Mortality in Elderly Patients with Dementia-Related Psychosis
- Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at increased risk of death. Analyses of 17 placebo-controlled trials (modal duration of 10 weeks), largely in patients taking atypical antipsychotic drugs, revealed a risk of death in drug-treated patients of 1.6 to 1.7 times the risk of death in placebo-treated patients. Over the course of a typical 10-week controlled trial, the rate of death in drug-treated patients was about 4.5%, compared to a rate of about 2.6% in the placebo group. Although the cases of death were varied, most of the deaths appeared to be either cardiovascular (e.g., heart failure, sudden death) or infectious (e.g., pneumonia) in nature. Observational studies suggest that, similar to atypical antipsychotic drugs, treatment with conventional antipsychotic drugs may increase mortality. The extent to which the findings of increased mortality in observational studies can be attributed to the antipsychotic drug as opposed to some characteristic(s) of the patients is not clear. ADASUVE is not approved for the treatment of elderly patients with dementia-related psychosis.
Neuroleptic Malignant Syndrome
- Antipsychotic drugs can cause a potentially fatal symptom complex termed Neuroleptic Malignant Syndrome (NMS). Clinical manifestations of NMS include hyperpyrexia, muscle rigidity, altered mental status, and autonomic instability (irregular pulse or blood pressure, tachycardia, diaphoresis, and cardiac dysrhythmia). Associated features can include elevated serum creatine phosphokinase (CPK) concentration, rhabdomyolysis, elevated serum and urine myoglobin concentration, and renal failure. NMS did not occur in the ADASUVE clinical program.
- The diagnostic evaluation of patients with this syndrome is complicated. It is important to consider the presence of other serious medical conditions (e.g., pneumonia, systemic infection, heat stroke, primary CNS pathology, central anticholinergic toxicity, extrapyramidal symptoms, or drug fever).
- The management of NMS should include: 1) immediate discontinuation of antipsychotic drugs and other drugs that may contribute to the underlying disorder, 2) intensive symptomatic treatment and medical monitoring, and 3) treatment of any concomitant serious medical problems. There is no general agreement about specific pharmacological treatment regimens for NMS.
- If a patient requires antipsychotic drug treatment after recovery from NMS, the potential reintroduction of drug therapy should be carefully considered. The patient should be carefully monitored, since recurrences of NMS have been reported.
Hypotension and Syncope
- ADASUVE can cause hypotension, orthostatic hypotension, and syncope. Use ADASUVE with caution in patients with known cardiovascular disease (history of myocardial infarction or ischemic heart disease, heart failure or conduction abnormalities), cerebrovascular disease, or conditions that would predispose patients to hypotension (dehydration, hypovolemia, or treatment with antihypertensive medications or other drugs that affect blood pressure or reduce heart rate).
- In the presence of severe hypotension requiring vasopressor therapy, the preferred drugs may be norepinephrine or phenylephrine. Epinephrine should not be used, because beta stimulation may worsen hypotension in the setting of ADASUVE-induced partial alpha blockade.
- In short-term (24-hour) placebo-controlled trials of patients with agitation associated with schizophrenia or bipolar I disorder, hypotension occurred in 0.4% and 0.8% in the ADASUVE 10 mg and placebo groups, respectively. There were no cases of orthostatic hypotension, postural symptoms, presyncope or syncope. A systolic blood pressure ≤ 90 mm Hg with a decrease of ≥ 20 mm Hg occurred in 1.5% and 0.8% of the ADASUVE 10 mg and placebo groups, respectively. A diastolic blood pressure ≤ 50 mm Hg with a decrease of ≥15 mm Hg occurred in 0.8% and 0.4% of the ADASUVE 10 mg and placebo groups, respectively.
- In 5 Phase 1 studies in normal volunteers, the incidence of hypotension was 3% and 0% in ADASUVE 10 mg and the placebo groups, respectively. The incidence of syncope or presyncope in normal volunteers was 2.3% and 0% in the ADASUVE and placebo groups, respectively. In normal volunteers, a systolic blood pressure ≤ 90 mm Hg with a decrease of ≥ 20 mm Hg occurred in 5.3% and 1.1% in the ADASUVE and placebo groups, respectively. A diastolic blood pressure ≤ 50 mm Hg with a decrease of ≥ 15 mm Hg occurred in 7.5% and 3.3% in the ADASUVE and placebo groups, respectively.
Seizures
- ADASUVE lowers the seizure threshold. Seizures have occurred in patients treated with oral loxapine. Seizures can occur in epileptic patients even during antiepileptic drug maintenance therapy. In short term (24 hour), placebo-controlled trials of ADASUVE, there were no reports of seizures.
Potential for Cognitive and Motor Impairment
- ADASUVE can impair judgment, thinking, and motor skills. In short-term, placebo-controlled trials, sedation and/or somnolence were reported in 12% and 10% in the ADASUVE and placebo groups, respectively. No patients discontinued treatment because of sedation or somnolence.
- The potential for cognitive and motor impairment is increased when ADASUVE is administered concurrently with other CNS depressants [see Drug Interactions (7.1)]. Caution patients about operating hazardous machinery, including automobiles, until they are reasonably certain that therapy with ADASUVE does not affect them adversely.
Cerebrovascular Reactions, Including Stroke, in Elderly Patients with Dementia-Related Psychosis
- In placebo-controlled trials with atypical antipsychotics in elderly patients with dementia-related psychosis, there was a higher incidence of cerebrovascular adverse reactions (stroke and transient ischemic attacks), including fatalities, compared to placebo-treated patients. ADASUVE is not approved for the treatment of patients with dementia-related psychosis [see BOXED WARNING and Warnings and Precautions (5.3)].
Anticholinergic Reactions Including Exacerbation of Glaucoma and Urinary Retention
- ADASUVE has anticholinergic activity, and it has the potential to cause anticholinergic adverse reactions including exacerbation of glaucoma or urinary retention. The concomitant use of other anticholinergic drugs (e.g., antiparkinson drugs) with ADASUVE could have additive effects.
Adverse Reactions
Clinical Trials Experience
- The following adverse reactions are discussed in more detail in other sections of the labeling:
- Hypersensitivity (serious skin reactions)
- Bronchospasm
- Increased Mortality in Elderly Patients with Dementia-Related Psychosis
- Neuroleptic Malignant Syndrome
- Hypotension and syncope
- Seizure
- Potential for Cognitive and Motor Impairment
- Cerebrovascular Reactions, Including Stroke, in Elderly Patients with Dementia-Related Psychosis
- Anticholinergic Reactions Including Exacerbation of Glaucoma and Urinary Retention
Clinical Trials Experience
- Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
- The following findings are based on pooled data from three short-term (24-hour), randomized, double-blind, placebo-controlled clinical trials (Studies 1, 2, and 3) of ADASUVE 10 mg in the treatment of patients with acute agitation associated with schizophrenia or bipolar I disorder. In the 3 trials, 259 patients received ADASUVE 10 mg, and 263 received placebo.
- Commonly Observed Adverse Reactions: In the 3 trials in acute agitation, the most common adverse reactions were dysgeusia, sedation, and throat irritation. These reactions occurred at a rate of at least 2% of the ADASUVE group and at a rate greater than in the placebo group. (Refer to Table 1).
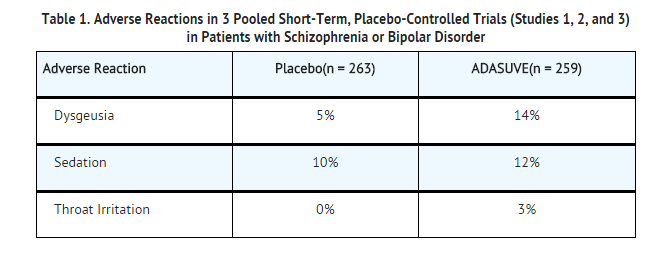
Airway Adverse Reactions in the 3 Trials in Acute Agitation
- Agitated patients with Schizophrenia or Bipolar Disorder: In the 3 short-term (24-hour), placebo-controlled trials in patients with agitation associated with schizophrenia or bipolar disorder (Studies 1, 2, and 3), bronchospasm (which includes reports of wheezing, shortness of breath and cough) occurred more frequently in the ADASUVE group, compared to the placebo group: 0% (0/263) in the placebo group and 0.8% (2/259) in the ADASUVE 10 mg group. One patient with schizophrenia, without a history of pulmonary disease, had significant bronchospasm requiring rescue treatment with a bronchodilator and oxygen.
Bronchospasm and Airway Adverse Reactions in Pulmonary Safety Trials
- Clinical pulmonary safety trials demonstrated that ADASUVE can cause bronchospasm as measured by FEV1, and as indicated by respiratory signs and symptoms in the trials. In addition, the trials demonstrated that patients with asthma or other pulmonary diseases, such as COPD are at increased risk of bronchospasm. The effect of ADASUVE on pulmonary function was evaluated in 3 randomized, double-blind, placebo-controlled clinical pulmonary safety trials in healthy volunteers, patients with asthma, and patients with COPD. Pulmonary function was assessed by serial FEV1 tests, and respiratory signs and symptoms were assessed. In the asthma and COPD trials, patients with respiratory symptoms or FEV1 decrease of ≥ 20% were administered rescue treatment with albuterol (metered dose inhaler or nebulizer) as required. These patients were not eligible for a second dose; however, they had continued FEV1 monitoring in the trial.
Healthy Volunteers: In the healthy volunteer crossover trial, 30 subjects received 2 doses of either ADASUVE or placebo 8 hours apart, and 2 doses of the alternate treatment at least 4 days later. The results for maximum decrease in FEV1 are presented in Table 2. No subjects in this trial developed airway related adverse reactions (cough, wheezing, chest tightness, or dyspnea).
Asthma Patients: In the asthma trial, 52 patients with mild-moderate persistent asthma (with FEV1 ≥ 60% of predicted) were randomized to treatment with 2 doses of ADASUVE 10 mg or placebo. The second dose was to be administered 10 hours after the first dose. Approximately 67% of these patients had a baseline FEV1 ≥ 80% of predicted. The remaining patients had an FEV1 60-80% of predicted. Nine patients (17%) were former smokers. As shown in Table 2 and Figure 7, there was a marked decrease in FEV1 immediately following the first dose (maximum mean decreases in FEV1 and % predicted FEV1 were 303 mL and 9.1%, respectively). Furthermore, the effect on FEV1 was greater following the second dose (maximum mean decreases in FEV1 and % predicted FEV1 were 537 mL and 14.7 %, respectively). Respiratory-related adverse reactions (bronchospasm, chest discomfort, cough, dyspnea, throat tightness, and wheezing) occurred in 54% of ADASUVE-treated patients and 12% of placebo-treated patients. There were no serious adverse events. Nine of 26 (35%) patients in the ADASUVE group, compared to one of 26 (4%) in the placebo group, did not receive a second dose of study medication, because they had a ≥ 20% decrease in FEV1 or they developed respiratory symptoms after the first dose. Rescue medication (albuterol via metered dose inhaler or nebulizer) was administered to 54% of patients in the ADASUVE group [7 patients (27%) after the first dose and 7 of the remaining 17 patients (41%) after the second dose] and 12% in the placebo group (1 patient after the first dose and 2 patients after the second dose).
COPD Patients: In the COPD trial, 53 patients with mild to severe COPD (with FEV1 ≥ 40% of predicted) were randomized to treatment with 2 doses of ADASUVE 10 mg or placebo. The second dose was to be administered 10 hours after the first dose. Approximately 57% of these patients had moderate COPD [Global Initiative for Chronic Obstructive Lung Disease (GOLD) Stage II]; 32% had severe disease (GOLD Stage III); and 11% had mild disease (GOLD Stage I). As illustrated in Table 2 there was a decrease in FEV1 soon after the first dose (maximum mean decreases in FEV1 and % predicted FEV1 were 96 mL and 3.5%, respectively), and the effect on FEV1 was greater following the second dose (maximum mean decreases in FEV1 and % predicted FEV1 were 125 mL and 4.5%, respectively). Respiratory adverse reactions occurred more frequently in the ADASUVE group (19%) than in the placebo group (11%). There were no serious adverse events. Seven of 25 (28%) patients in the ADASUVE group and 1of 27 (4%) in the placebo group did not receive a second dose of study medication because of a ≥ 20% decrease in FEV1 or the development of respiratory symptoms after the first dose. Rescue medication (albuterol via MDI or nebulizer) was administered to 23% of patients in the ADASUVE group: 8% of patients after the first dose and 21% of patients after the second dose, and to 15% of patients in the placebo group.
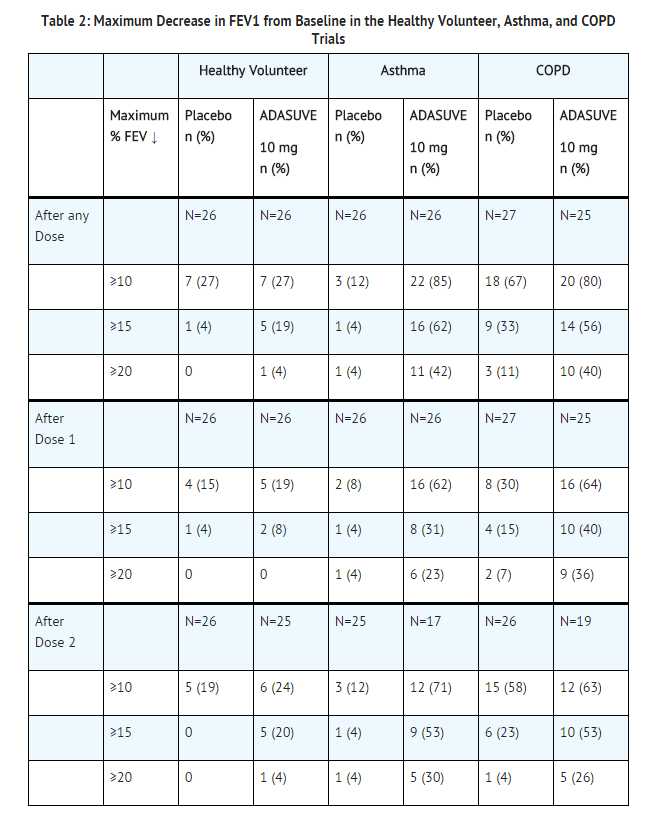
- FEV1 categories are cumulative; i.e. a subject with a maximum decrease of 21% is included in all 3 categories. Patients with a ≥ 20% decrease in FEV1 did not receive a second dose of study drug.
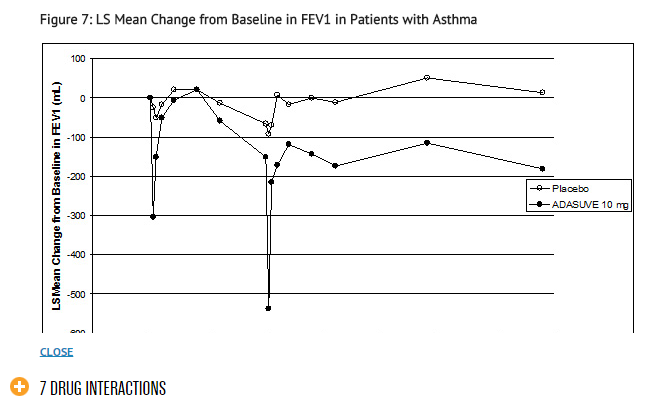
- Patients with a ≥ 20% decrease in FEV1 did not receive a second dose of study drug and are not included in the curves beyond hour 10.
Extrapyramidal Symptoms (EPS): Extrapyramidal reactions have occurred during the administration of oral loxapine. In most patients, these reactions involved parkinsonian symptoms such as tremor, rigidity, and masked facies. Akathisia(motor restlessness) has also occurred.
- In the 3 short-term (24-hour), placebo-controlled trials of ADASUVE in 259 patients with agitation associated with schizophrenia or bipolar disorder, extrapyramidal reactions occurred. One patient (0.4%) treated with ADASUVE developed neck dystonia and oculogyration. The incidence of akathisia was 0% and 0.4% in the placebo and ADASUVE groups, respectively.
Dystonia (Antipsychotic Class Effect: Symptoms of dystonia, prolonged abnormal contractions of muscle groups, may occur in susceptible individuals during treatment with ADASUVE. Dystonic symptoms include spasm of the neck muscles, sometimes progressing to tightness of the throat, difficulty swallowing or breathing, and/or protrusion of the tongue.
- Acute dystonia tends to be dose-related, but can occur at low doses, and occurs more frequently with first generation antipsychotic drugs such as ADASUVE. The risk is greater in males and younger age groups.
Cardiovascular Reactions: Tachycardia, hypotension, hypertension, orthostatic hypotension, lightheadedness, and syncope have been reported with oral administration of loxapine.
Postmarketing Experience
There is limited information regarding Postmarketing Experience of Loxapine (inhalation) in the drug label.
Drug Interactions
CNS Depressants
- ADASUVE is a central nervous system (CNS) depressant. The concurrent use of ADASUVE with other CNS depressants (e.g., alcohol, opioid analgesics, benzodiazepines, tricyclic antidepressants, general anesthetics, phenothiazines, sedative/hypnotics, muscle relaxants, and/or illicit CNS depressants) can increase the risk of respiratory depression, hypotension, profound sedation, and syncope. Therefore, consider reducing the dose of CNS depressants if used concomitantly with ADASUVE.
Anticholinergic Drugs
- ADASUVE has anticholinergic activity. The concomitant use of ADASUVE and other anticholinergic drugs can increase the risk of anticholinergic adverse reactions including exacerbation of glaucoma and urinary retention.
Use in Specific Populations
Pregnancy
- Pregnancy Category
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Loxapine (inhalation) in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Loxapine (inhalation) during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Loxapine (inhalation) with respect to nursing mothers.
Pediatric Use
There is no FDA guidance on the use of Loxapine (inhalation) with respect to pediatric patients.
Geriatic Use
There is no FDA guidance on the use of Loxapine (inhalation) with respect to geriatric patients.
Gender
There is no FDA guidance on the use of Loxapine (inhalation) with respect to specific gender populations.
Race
There is no FDA guidance on the use of Loxapine (inhalation) with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Loxapine (inhalation) in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Loxapine (inhalation) in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Loxapine (inhalation) in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Loxapine (inhalation) in patients who are immunocompromised.
Administration and Monitoring
Administration
- Inhalation
Monitoring
There is limited information regarding Monitoring of Loxapine (inhalation) in the drug label.
IV Compatibility
There is limited information regarding IV Compatibility of Loxapine (inhalation) in the drug label.
Overdosage
Signs and Symptoms of Overdosage
- As would be expected from the pharmacologic actions of loxapine, the clinical findings may include CNS depression, unconsciousness, profound hypotension, respiratory depression, extrapyramidal symptoms, and seizure.
Management of Overdosage
- For the most up to date information on the management of ADASUVE overdosage, contact a certified poison control center (1-800-222-1222 or www.poison.org). Provide supportive care including close medical supervision and monitoring. Treatment should consist of general measures employed in the management of overdosage with any drug. Consider the possibility of multiple drug overdosage. Ensure an adequate airway, oxygenation, and ventilation. Monitor cardiac rhythm and vital signs. Use supportive and symptomatic measures.
Pharmacology
Mechanism of Action
- The mechanism of action of loxapine in the treatment of agitation associated with schizophrenia is unknown. However, its efficacy could be mediated through a combination of antagonism of central dopamine D2 and serotonin 5-HT2A receptors. The mechanism of action of loxapine in the treatment of agitation associated with bipolar I disorder is unknown.
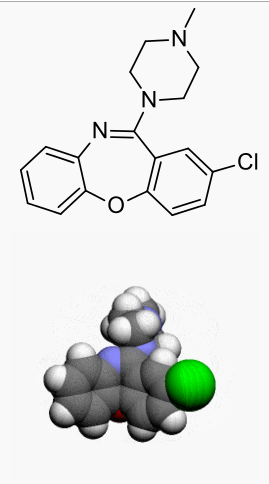
Structure
- ADASUVE, a typical antipsychotic, is an inhalation powder of loxapine supplied in a single-use, disposable inhaler containing 10 mg of loxapine base. ADASUVE is a drug-device combination product.
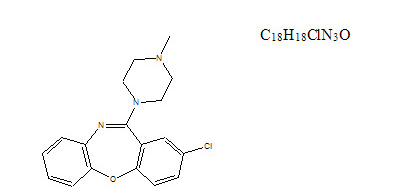
Active Ingredient: Loxapine (base). Loxapine, a dibenzoxazepine compound, represents a subclass of tricyclic antipsychotic agents, chemically distinct from the thioxanthenes, butyrophenones, and phenothiazines. Chemically, it is 2-Chloro-11-(4-methyl-1-piperazinyl) dibenz [b,f] [1,4] oxazepine.
- ADASUVE is a single-use, drug-device combination product that provides rapid systemic delivery by inhalation of a thermally-generated aerosol of loxapine. Oral inhalation through the product initiates the controlled rapid heating of a thin film of excipient-free loxapine to form a thermally-generated drug vapor. The vapor condenses into aerosol particles that are dispersed into the airstream created by the patient inhaling through the mouthpiece.
- Each product is packaged inside a sealed foil pouch. The product is a white to off-white plastic unit, with a mouthpiece on one end and a pull-tab protruding from the other end.
- Removal of a pull-tab from the product renders it ready for use, as indicated by illumination of a green light. After inhalation through the mouthpiece, successful dosing is signaled by the green light turning off.
- Under standardized in vitro test conditions, ADASUVE, 10 mg delivers 9.1 mg of loxapine out of the mouthpiece.
Pharmacodynamics
- Loxapine acts as an antagonist at central serotonin and dopamine receptors, with high affinity for serotonin 5-HT2A and dopamine D1, D2, D3, and D4 receptors (Ki values of 2 nM, 18 nM, 10 nM, 21 nM, 9 nM, respectively). Some of the adverse effects of loxapine may be related to the antagonism of histamine H1 (somnolence), muscarinic M1 (anticholinergic), and adrenergic α2 (orthostatic hypotension) receptors (Ki values of 15 nM, 117 nM and 250 nM, respectively).
Thorough QTc Study
- ADASUVE did not prolong the QTc interval. The effect of ADASUVE on QTc prolongation was evaluated in a randomized, double-blinded, positive- (moxifloxacin 400 mg) and placebo-controlled parallel study in healthy subjects. A total of 48 healthy subjects were administered ADASUVE 10 mg. In this study with a demonstrated ability to detect small effects, the upper bound of the 90% confidence interval (CI) for the largest placebo-adjusted, baseline-corrected QTc based on individual correction method was below 10 milliseconds, the threshold for regulatory concern.
Pharmacokinetics
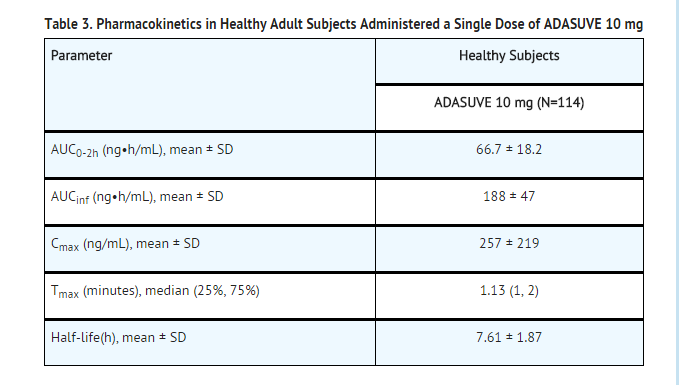
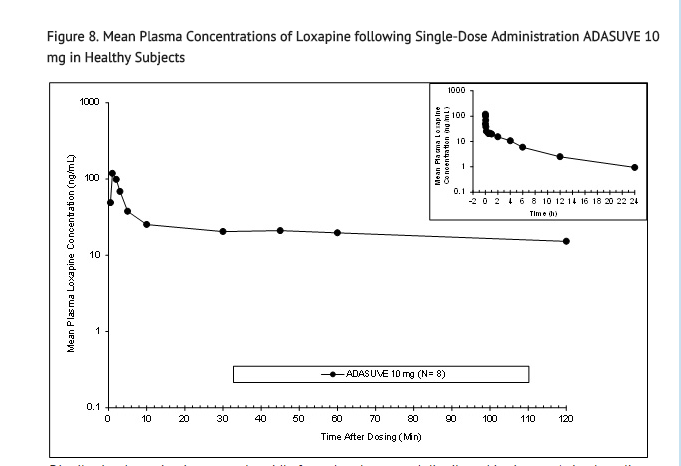
Absorption: The single-dose pharmacokinetic parameters of loxapine following administration of single doses of ADASUVE 10 mg in healthy adult subjects are presented in Table 3 and Figure 8.
- Administration of ADASUVE resulted in rapid absorption of loxapine, with a median time of maximum plasma concentration (Tmax) of 2 minutes. Loxapine exposure in the first 2 hours after administration (AUC0-2h) was 66.7 ng•h/mL for the 10 mg dose. As a consequence of the very rapid absorption of loxapine after oral inhalation, there is substantial variability in the early plasma concentrations of loxapine. The mean plasma loxapine concentrations following administration of ADASUVE were linear over the clinical dose range. AUC0-2h, AUCinf, and Cmax increased in a dose-dependent manner.
Distribution: Loxapine is removed rapidly from the plasma and distributed in tissues. Animal studies following oral administration suggest an initial preferential distribution in the lungs, brain, spleen, heart, and kidney. Loxapine is 96.6% bound to human plasma proteins.
Metabolism: Loxapine is metabolized extensively in the liver following oral administration, with multiple metabolites formed. The main metabolic pathways include: 1) hydroxylation to form 8-OH-loxapine by CYP1A2 and 7-OH-loxapine by CYP3A4 and CYP2D6, 2) N-oxidation to form loxapine N-oxide by flavanoid monoamine oxidases (FMOs), and 3) de-methylation to form amoxapine. Because there are multiple metabolic pathways, the risk of metabolic interactions caused by an effect on an individual isoform is minimal. For ADASUVE, the order of metabolites observed in humans (based on systemic exposure) was 8-OH-loxapine >> loxapine N-oxide, 7-OH-loxapine > amoxapine. Plasma levels of 8-OH-loxapine are similar to those of the parent compound.
Excretion: Excretion occurs mainly in the first 24 hours. Metabolites are excreted in the urine in the form of conjugates and in the feces unconjugated. The terminal elimination half-life (T1/2) ranged from 6 to 8 hours.
Transporter Interaction: In vitro studies indicated that loxapine was not a substrate for p-glycoprotein (P-gp): however, loxapine inhibited P-gp.
Special Populations:
Pharmacokinetics in Smokers: Loxapine exposures in nonsmokers and smokers are similar, with geometric mean ratios of 92%, 85%, and 99% for AUC0-2h, AUCinf, and Cmax respectively. No dosage adjustment is recommended based on smoking status.
Demographic Effects: There were no clinically significant differences in loxapine pharmacokinetics following administration of ADASUVE in subgroups based on age, weight, body mass index, gender, or race.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis: No adequate studies have been conducted.
Mutagenesis: Loxapine did not cause mutation or chromosomal aberration when tested in vitro and in vivo. Loxapine was negative in the Ames gene mutation assay, the human peripheral blood lymphocyte chromosomal aberration assay, and in the in vivo mouse bone marrow micronucleus assay up to 40 mg/kg (20-fold the MRHD on mg/m2 basis).
- Loxapine metabolite 8-OH-loxapine was not mutagenic in the in vitro Ames reverse mutation assay and was not clastogenic in the in vitro human peripheral blood lymphocyte chromosomal aberration assay.
Impairment of Fertility: Loxapine had no effects on fertility or early embryonic development in male rats or in male and female rabbits following oral administration. Mating was decreased in female rats because these animals were in persistent diestrus, an expected pharmacologic effect for this class of compounds. This occurred at doses approximately 0.2- and 1-fold the MRHD of 10 mg/day on a mg/m2 basis.
Animal Toxicology and/or Pharmacology
- In the rat, minimal and reversible squamous metaplasia of the larynx was observed after daily inhalation exposure of loxapine for 14 days at 1.7 to 13 mg/kg/day (approximately 2- to 13-fold the MRHD of 10 mg/day on a mg/m2 basis, respectively). This finding was considered a nonspecific particle impaction effect. Mammary hyperplasia in males and females and ovarian follicular cysts and mucification of vaginal epithelium in female rats were observed at all doses, with partial or complete recovery at the end of 14 days of treatment. In the dog, no effects on the respiratory tract or reproductive tissues were observed after inhalation exposure to loxapine for 28 days at doses up to 1.8 mg/kg/day (approximately 6-fold the MRHD of 10 mg/day on a mg/m2 basis).
Clinical Studies
- The efficacy of ADASUVE 10 mg in the acute treatment of agitation associated with schizophrenia or bipolar I disorder was established in two short-term (24-hour), randomized, double-blind, placebo-controlled, fixed-dose trials. Study 1 included 344 patients who met DSM-IV criteria for schizophrenia. Study 2 included 314 patients who met DSM-IV criteria for bipolar I disorder, manic or mixed episodes with or without psychotic features.
- Patients were judged by the clinical investigators to be clinically agitated, with a level of agitation that met or exceeded a specific severity threshold as measured by the Positive and Negative Syndrome Scale-Excited Component (PEC). The PEC is an investigator-rated instrument consisting of 5 items: poor impulse control, tension, hostility, uncooperativeness, and excitement. Each item is scored on a scale from 1 to 7 (1 = absent, 4 = moderate, 7 = extreme). Thus, the total PEC score can range from 5 to 35. For enrollment in the studies, patients had to have a PEC score of ≥ 14, with at least one individual item score ≥ 4.
- Patients whose agitation was related to acute alcohol or drug intoxication were excluded. Patients with clinically significant acute or chronic pulmonary disease (e.g., asthma, COPD, chronic bronchitis, and emphysema) were excluded from the trials.
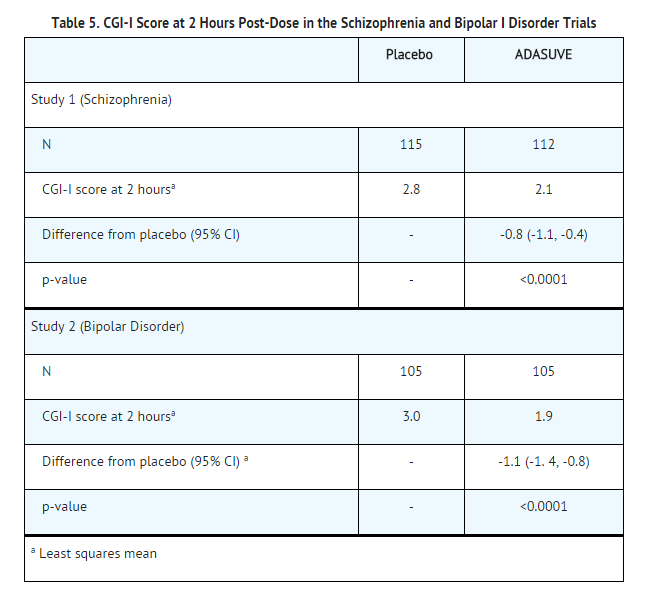
- The primary efficacy endpoint in both trials was the mean change from baseline in the PEC score, assessed 2 hours following dosing. The key secondary endpoint was the mean Clinical Global Impression Improvement (CGI-I) Scale score at two hours. The CGI-I is an investigator-rated global assessment of symptom improvement, scored on a scale of 1 to 7: 1 = very much improved; 4 = no change from baseline; 7 = very much worse.
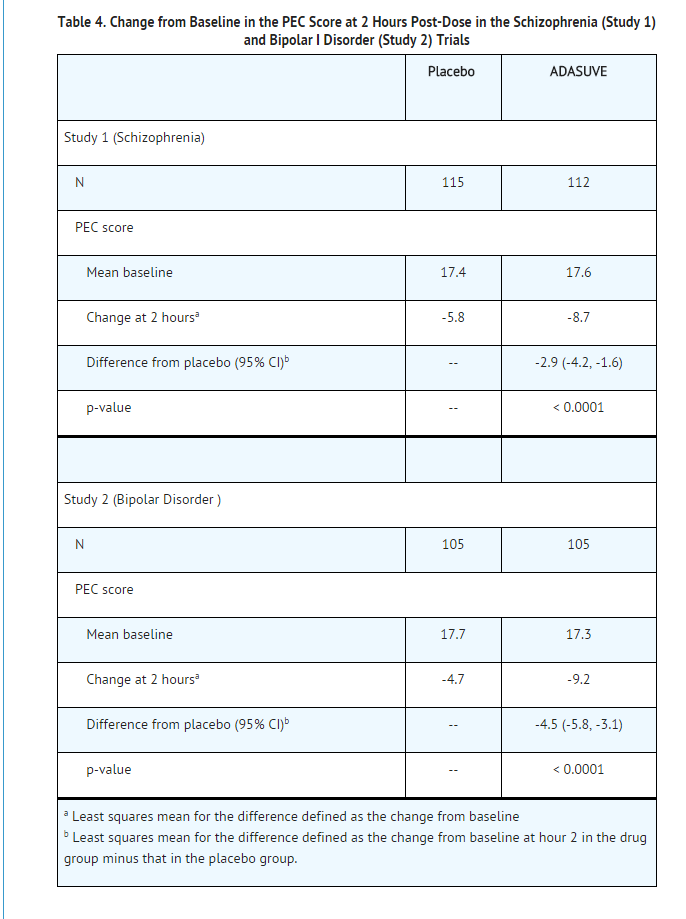
- In both studies, mean baseline PEC scores were similar in all treatment groups, averaging 17.3 to 17.7 (Table 4), with individual patient scores ranging from 14 to 31, indicating predominantly moderate levels of agitation. The mean baseline Clinical Global Impression Severity Scale (CGI-S) score in both studies was 4 (moderately ill). In Study 2, 69% of patients had a current manic episode, and 31% had a mixed/manic episode.
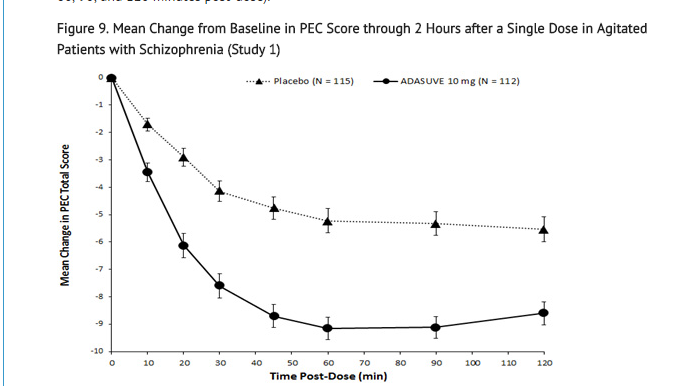
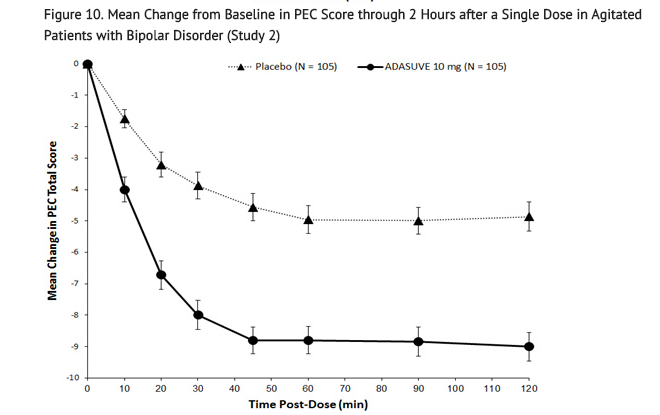
- In Studies 1 and 2, treatment with ADASUVE was statistically significantly superior to placebo on the mean change in PEC score at 2 hours (Table 4). In both studies, the effect of ADASUVE was apparent at 10 minutes following dosing (Figures 9 and 10).
- Examination of population subsets (age, race, and gender) on the primary endpoint did not reveal any differential responsiveness on the basis of these subgroupings.
- Figures 9 and 10 show the decreases in PEC score at each time point assessed in the trials. In both trials, the decrease in agitation with ADASUVE was apparent at each time point tested (10, 20, 30, 45, 60, 90, and 120 minutes post-dose).
- The results of the secondary endpoint, CGI-I scores, are shown in Table 5.
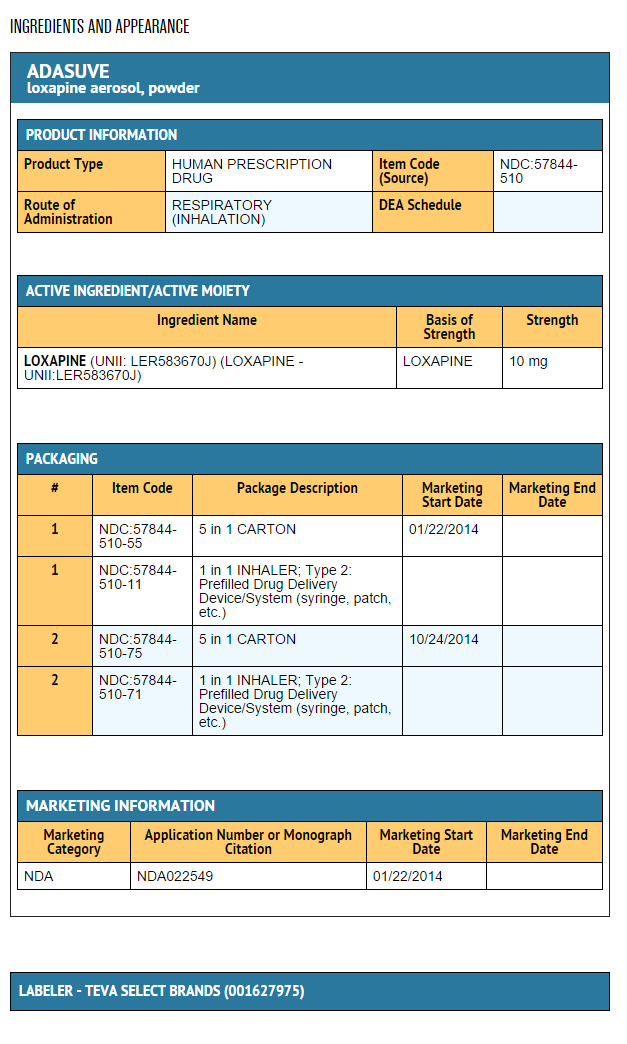
How Supplied
- ADASUVE® (loxapine) inhalation powder is supplied as:
- ADASUVE 10 mg (NDC 57844-510-11) is a single-use, disposable inhaler containing 10 mg of loxapine, provided in a sealed foil pouch. ADASUVE, 10 mg is supplied in a carton of 5 units per carton (NDC 57844-510-55).
Storage
- Store ADASUVE at room temperature, 15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature].
Images
Drug Images
{{#ask: Page Name::Loxapine (inhalation) |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel


{{#ask: Label Page::Loxapine (inhalation) |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
Bronchospasm
- Advise patients and caregivers that there is a risk of bronchospasm. Advise patients to inform their healthcare professional if they develop any breathing problems such as wheezing, shortness of breath, chest tightness, or cough following treatment with ADASUVE.
Interference with Cognitive and Motor Performance
- Caution patients and caregivers about performing activities requiring mental alertness, such as operating hazardous machinery or operating a motor vehicle, until they are reasonably certain that ADASUVE has not affected them adversely.
- Caution patients and caregivers about the potential for sedation, especially when used concurrently with other CNS depressants (e.g., alcohol, opioid analgesics, benzodiazepines, tricyclic antidepressants, general anesthetics, phenothiazines, sedative/hypnotics, muscle relaxants, and/or illicit CNS depressants).
Neuroleptic Malignant Syndrome
- Patients and caregivers should be counseled that a potentially fatal symptom complex sometimes referred to as NMS has been reported in association with administration of antipsychotic drugs. Signs and symptoms of NMS include hyperpyrexia, muscle rigidity, altered mental status, and evidence of autonomic instability (irregular pulse or blood pressure, tachycardia, diaphoresis, and cardiac dysrhythmia).
Hypotension and Syncope
- Advise patients and caregivers of the risk of hypotension or orthostatic hypotension (symptoms include feeling dizzy or lightheaded upon standing).
Anticholinergic Reactions
- Counsel patients and caregivers about the potential risks of anticholinergic reactions, such as exacerbation of glaucoma and urinary retention.
Pregnancy
- Counsel patients and caregivers regarding the potential risk to the fetus or neonate.
Nursing Mothers
- Counsel patients and caregivers regarding the potential risk to the infant.
Precautions with Alcohol
- Alcohol-Loxapine (inhalation) interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- ADASUVE®[1]
Look-Alike Drug Names
- A® — B®[2]
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ "loxapine aerosol, powder".
- ↑ "http://www.ismp.org". External link in
|title=(help)
{{#subobject:
|Page Name=Loxapine (inhalation)
|Pill Name=No image.jpg
|Drug Name=
|Pill Ingred=|+sep=;
|Pill Imprint=
|Pill Dosage={{{dosageValue}}} {{{dosageUnit}}}
|Pill Color=|+sep=;
|Pill Shape=
|Pill Size (mm)=
|Pill Scoring=
|Pill Image=
|Drug Author=
|NDC=
}}
{{#subobject:
|Label Page=Loxapine (inhalation) |Label Name=Loxapine (inhalation)11.png
}}
{{#subobject:
|Label Page=Loxapine (inhalation) |Label Name=Loxapine (inhalation)11.png
}}
 KSF
KSF