Abdominal aortic aneurysm
 From Citizendium - Reading time: 5 min
From Citizendium - Reading time: 5 min
In health care, an abdominal aortic aneurysm is "an abnormal balloon- or sac-like dilatation in the wall of the abdominal aorta which gives rise to the visceral, the parietal, and the terminal (iliac) branches below the aortic hiatus at the diaphragm."[1]
Risk factors[edit]
- smoking
- syphilis
- tuberculosis
Diagnosis[edit]
Various methods of diagnostic imaging can be used to measure the diameter of the aorta.[2]
Screening[edit]
A clinical practice guideline by the U.S. Preventive Services Task Force (USPSTF) 'recommends one-time screening for abdominal aortic aneurysm (AAA) by ultrasonography in men age 65 to 75 years who have ever smoked'.[3][4] This is a grade B recommendation. An re-analysis of the meta-analysis estimated a number needed to screen of approximately 850 patients.[5]
The largest of the randomized controlled trials on which this guideline was based studied a screening program that consisted of[6]:
- Screening men ages 65-74 years (not restricted to ever smokers). 'Men in whom abdominal aortic aneurysms (> or =3 cm in diameter) were detected were followed-up... Patients with an aortic diameter of 3·0–4·4 cm were rescanned at yearly intervals, whereas those with an aortic diameter of 4·5–5·4 cm were rescanned at 3-monthly intervals ... Surgery was considered on specific criteria (diameter > or =5.5 cm, expansion > or =1 cm per year, symptoms)'.
This trial reported significant short[6] ( number needed to screen after 4 years of approximately 590 to prevent nonfatal ruptured AAA plus AAA-related deaths[7]) and long term[8] ( number needed to screen after 7 years of approximately 280 to prevent nonfatal ruptured AAA plus AAA-related deaths) benefit and cost effectiveness.[9] Subsequent randomized controlled trials also found benefit:
- number needed to screen after 4 years of 300[10]
- number needed to screen after and after 7 years of 563 (calculation).[11]
More recently, a clinical prediction rule may help identify patients for screening.[12]
MEDICARE criteria for screening[edit]
Effective January 1, 2007, provisions of the SAAAVE Act (Screening Abdominal Aortic Aneurysm Very Efficiently) now provide a free, one-time, ultrasound AAA screening benefit for those qualified seniors. Men who have smoked at least 100 cigarettes during their life, and men and women with a family history of AAA qualify for the one-time ultrasound screening.
Enrollees must visit their healthcare professional for their Welcome to Medicare physical within six months of enrollment in order to qualify for the free screening.
The Welcome to Medicare Physical Exam must be completed within the first six months of Medicare eligibility, but there is no published time limit thereafter for completion of the AAA screening. Providers who perform the physical and order the AAA screening need to document the AAA risk factors.[13]
Differential diagnosis[edit]
Potential comorbidities[edit]
Treatment[edit]
Indications for surgery in patients without symptoms are:[14]
- size greater than 5.5 cm in diameter
- "becomes tender"
- grows more than 1 cm in diameter per year
Surgical repair[edit]
Repair should be considered for symptomatic aneurysms or those larger than 5.5 cm according to a systematic review[15] of randomized controlled trials.[16]>[17]
Endovascular repair[edit]
| Patients | Intervention | Comparison | Outcome | Results | |
|---|---|---|---|---|---|
| DREAM[20] (2010) |
Aneurysms > 5.0 cm Mean age 70 |
Endovascular | Open repair | Mortality at 6 yrs | Endo=31% Open=30% |
| EVAR-1[18] (2010) |
Aneurysms > 5.5 cm Mean age 74 |
Endovascular | Open repair | Mortality at 6 yrs | Endo=42% Open=42% |
| EVAR-2[19] (2010) |
Aneurysms > 5.5 cm Mean age 77 Unfit for open repair |
Endovascular | None | Mortality at 3 yrs | Endo=74% None=77% |
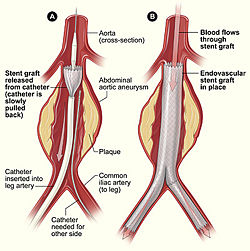
Endovascular repair (i.e., inserting a stent or patch) is a less invasive procedure that may be used when the renal arteries are not part of the aneurysm.[21] Endovascular repair has improved short term outcomes.[22] However, it has similar outcomes as compared to open surgery at two years[23] and six years[20].
In a cohort study, endovascular repair may have improved outcomes as compared to open repair.[21]
Complications[edit]
Acute kidney injury[edit]
Acute kidney injury is uncommon (less than 2% of patients) if the aneurysm is infrarenal and reimplantation of the renal arteries is not needed.[22]
Prognosis[edit]
Attribution[edit]
- Some content on this page may previously have appeared on Wikipedia.
References[edit]
- ↑ Anonymous (2025), Abdominal aortic aneurysm (English). Medical Subject Headings. U.S. National Library of Medicine.
- ↑ Long A, Rouet L, Lindholt JS, Allaire E (2012). "Measuring the maximum diameter of native abdominal aortic aneurysms: review and critical analysis.". Eur J Vasc Endovasc Surg 43 (5): 515-24. DOI:10.1016/j.ejvs.2012.01.018. PMID 22336051. Research Blogging.
- ↑ U.S. Preventive Services Task Force (2005). "Screening for abdominal aortic aneurysm: recommendation statement". Ann. Intern. Med. 142 (3): 198-202. PMID 15684208. [e]
- ↑ Fleming C, Whitlock EP, Beil TL, Lederle FA (2005). "Screening for abdominal aortic aneurysm: a best-evidence systematic review for the U.S. Preventive Services Task Force". Ann. Intern. Med. 142 (3): 203-11. PMID 15684209. [e] ACP Journal Club
- ↑ Cinà CS, Devereaux PJ (2005). "Review: population-based screening for abdominal aortic aneurysm reduces cause-specific mortality in older men". ACP J. Club 143 (1): 11. PMID 15989299. [e]
- ↑ 6.0 6.1 Ashton HA, Buxton MJ, Day NE, et al (2002). "The Multicentre Aneurysm Screening Study (MASS) into the effect of abdominal aortic aneurysm screening on mortality in men: a randomised controlled trial". Lancet 360 (9345): 1531-9. PMID 12443589. [e] ACP Journal Club
- ↑ Cina CS (2003). "Screening for abdominal aortic aneurysm reduced death from AAA in older men". ACP J. Club 138 (3): 66. PMID 12725621. [e]
- ↑ Kim LG, P Scott RA, Ashton HA, Thompson SG (2007). "A sustained mortality benefit from screening for abdominal aortic aneurysm". Ann. Intern. Med. 146 (10): 699-706. PMID 17502630. [e]
- ↑ Multicentre Aneurysm Screening Study Group (2002). "Multicentre aneurysm screening study (MASS): cost effectiveness analysis of screening for abdominal aortic aneurysms based on four year results from randomized controlled trial". BMJ 325 (7373): 1135. PMID 12433761. [e] ACP Journal Club
- ↑ Lindholt JS, Juul S, Fasting H, Henneberg EW (2005). "Screening for abdominal aortic aneurysms: single centre randomised controlled trial". BMJ 330 (7494): 750. DOI:10.1136/bmj.38369.620162.82. PMID 15757960. Research Blogging. ACP Journal Club
- ↑ Ashton HA, Gao L, Kim LG, Druce PS, Thompson SG, Scott RA (2007). "Fifteen-year follow-up of a randomized clinical trial of ultrasonographic screening for abdominal aortic aneurysms". The British journal of surgery 94 (6): 696-701. DOI:10.1002/bjs.5780. PMID 17514666. Research Blogging.
- ↑ Greco G, Egorova NN, Gelijns AC, Moskowitz AJ, Manganaro AJ, Zwolak RM et al. (2010). "Development of a novel scoring tool for the identification of large ≥5 cm abdominal aortic aneurysms.". Ann Surg 252 (4): 675-82. DOI:10.1097/SLA.0b013e3181f621c8. PMID 20881774. Research Blogging.
- ↑ Society for Vascular Surgery
- ↑ Greenhalgh RM, Powell JT (January 2008). "Endovascular repair of abdominal aortic aneurysm". N. Engl. J. Med. 358 (5): 494–501. DOI:10.1056/NEJMct0707524. PMID 18234753. Research Blogging.
- ↑ Lederle FA, Kane RL, MacDonald R, Wilt TJ. Systematic review: repair of unruptured abdominal aortic aneurysm. Ann Intern Med. 2007 May 15;146(10):735-41. PMID 17502634
- ↑ Lederle FA, Wilson SE, Johnson GR, et al. (May 2002). "Immediate repair compared with surveillance of small abdominal aortic aneurysms". N. Engl. J. Med. 346 (19): 1437–44. DOI:10.1056/NEJMoa012573. PMID 12000813. Research Blogging.
- ↑ United Kingdom Small Aneurysm Trial Participants (May 2002). "Long-term outcomes of immediate repair compared with surveillance of small abdominal aortic aneurysms". N. Engl. J. Med. 346 (19): 1445–52. DOI:10.1056/NEJMoa013527. PMID 12000814. Research Blogging.
- ↑ 18.0 18.1 United Kingdom EVAR Trial Investigators. Greenhalgh RM, Brown LC, Powell JT, Thompson SG, Epstein D et al. (2010). "Endovascular versus open repair of abdominal aortic aneurysm.". N Engl J Med 362 (20): 1863-71. DOI:10.1056/NEJMoa0909305. PMID 20382983. Research Blogging.
- ↑ 19.0 19.1 United Kingdom EVAR Trial Investigators. Greenhalgh RM, Brown LC, Powell JT, Thompson SG, Epstein D (2010). "Endovascular repair of aortic aneurysm in patients physically ineligible for open repair.". N Engl J Med 362 (20): 1872-80. DOI:10.1056/NEJMoa0911056. PMID 20382982. Research Blogging.
- ↑ 20.0 20.1 20.2 De Bruin JL, Baas AF, Buth J, Prinssen M, Verhoeven EL, Cuypers PW et al. (2010). "Long-term outcome of open or endovascular repair of abdominal aortic aneurysm.". N Engl J Med 362 (20): 1881-9. DOI:10.1056/NEJMoa0909499. PMID 20484396. Research Blogging.
- ↑ 21.0 21.1 Jackson RS, Chang DC, Freischlag JA (2012). "Comparison of long-term survival after open vs endovascular repair of intact abdominal aortic aneurysm among Medicare beneficiaries.". JAMA 307 (15): 1621-8. DOI:10.1001/jama.2012.453. PMID 22511690. Research Blogging.
- ↑ 22.0 22.1 Prinssen M, Verhoeven EL, Buth J, et al. (October 2004). "A randomized trial comparing conventional and endovascular repair of abdominal aortic aneurysms". N. Engl. J. Med. 351 (16): 1607–18. DOI:10.1056/NEJMoa042002. PMID 15483279. Research Blogging.
- ↑ Blankensteijn JD, de Jong SE, Prinssen M, et al. (June 2005). "Two-year outcomes after conventional or endovascular repair of abdominal aortic aneurysms". N. Engl. J. Med. 352 (23): 2398–405. DOI:10.1056/NEJMoa051255. PMID 15944424. Research Blogging.
 KSF
KSF