|
|
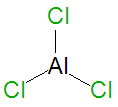
| Aluminium trichloride
|
| IUPAC name:
|
trichloroalumane
|
| Synonyms:
|
aluminium chloride,
|
| Formula:
|
AlCl3
|
|
|
| Uses:
|
catalyst, astringent
|
|
| Properties:
|
pyrophoric Lewis acid
|
|
| Hazards:
|
spontaneously ignites in air
|
|
| Mass (g/mol):
|
CAS #:
|
| 133.340538
|
79156-75-5
|
|
Aluminium trichloride, AlCl3, is an important industrial chemical catalyst, especially for polymerization reactions. It is a pyrophoric Lewis acid chemical compound which instantly ignites when it is exposed to air. It is a highly reactive compound that can also be used as a chemical promoter in chelation-controlled reactions. It is also an astringent, used medically for athlete's foot hyperhidrosis therapy.[1]
References[edit]
 From Citizendium - Reading time: 1 min
From Citizendium - Reading time: 1 min
 KSF
KSF