Antoine-Laurent Lavoisier
 From Citizendium - Reading time: 10 min
From Citizendium - Reading time: 10 min

Jacques-Louis David: Antoine-Laurent Lavoisier and his wife (Marie-Anne-Pierrette Paulze (1758-1836) in 1788
Antoine-Laurent Lavoisier (Paris, August 26, 1743 – Paris, May 8, 1794) was one of the major founders of modern chemistry. He performed experiments on the chemical reactivity of oxygen, recognizing it is a chemical element, thus rejecting the phlogiston hypothesis. He confirmed the earlier discovery of Henry Cavendish that the reaction of hydrogen with oxygen gives pure water and he gave the correct interpretation of this reaction. Lavoisier was also one of the authors of the modern system for naming chemical substances. He propagated the law of conservation of mass—in Europe sometimes referred to as "Lavoisier's law".
Having served as a tax collector before the French Revolution, he was guillotined together with 27 other tax collectors during the reign of terror.
Biography[edit]
Lavoisier was the oldest child and only son of a wealthy upper-middle class family; his only sister died at the age of fifteen. As a boy he was unusually inquisitive and loved to read and study. He went to the the prestigious Collège des Quatre-Nations, founded by Cardinal Mazarin, where he was taught humanities, mathematics, and sciences. After graduation, he enrolled as a law student at the law faculty of the University of Paris. Since this study was easy for him, he had much time to follow lectures on chemistry and physics and to do laboratory work under the tutelage of leading natural philosophers.
After obtaining his law degree in 1763, Lavoisier followed in his father's and his maternal grandfather's footsteps and became member of the Order of Barristers, associated with the High Court (Parlement de Paris) of Paris. However, Lavoisier did not start a law practice, but instead began pursuing scientific research that in 1768 gained him admission into one of France's most prestigious societies, the Académie des sciences in Paris.
When Lavoisier was five years old his mother died and left him a considerable inheritance, that he, shortly before entering the Academy of Sciences in 1768, used to purchase an interest in the Ferme générale (General Farm). The Ferme générale was, under the Ancien Régime, a franchised customs and excise operation which collected duties on behalf of the King, under 6-year contracts. The major tax collectors in that tax farming system were known as the fermiers généraux (general farmers). They collected certain sales and excise taxes, such as those on salt and tobacco. At the beginning of each financial cycle the tax farmers lent money to the government and were subsequently reimbursed through tax collections. Lavoisier spent considerable part of his time "farming" taxes, and he was richly rewarded for his efforts. In fact, the proceeds enabled him to live a comfortable life, equip a laboratory, and hire laboratory assistants. Although chemistry was Lavoisier's passion, throughout his life he devoted much of his time to financial and administrative affairs other than his tax business.
Three years after joining the General Farm, in 1771, Lavoisier married Marie-Anne Pierrette Paulze, the 13-year-old daughter of one of his colleagues at the Farm. Although not educated in science, Marie-Anne was an intelligent young woman. As Marie-Anne and Lavoisier had no children, Marie-Anne was able to devote her attentions to helping her husband in his research, and she soon became widely regarded as a valuable laboratory assistant and hostess. She knew English, which Lavoisier never did, and translated chemical works for him, for instance the works of Joseph Priestley. She employed her drawing talent to record the research conducted in the laboratory and to prepare engravings of apparatus for publications. In the laboratory she often recorded results that the experimenters dictated to her, and when Lavoisier announced his new theories she played an active role in campaigning for their acceptance. About 10 years after Lavoisier's death Marie-Anne married the American born Count Rumford (Benjamin Thompson).

Part of the "Advertisement" in Vol. 1 of the English translation of Lavoisier's Elements of Chemistry.[1]
Lavoisier also performed administrative duties within the Academy of Sciences and for other government agencies during the final years of the monarchy and the early years of the French Revolution. From 1775 to 1792 he served as a director of the French Gunpowder Administration and succeeded in making France self-sufficient in this critical military material. He developed a new method for the production of salpeter (potassium nitrate), an important ingredient of gun powder. He also conducted extensive experiments on agricultural production, advised the government on financial affairs and banking, and served on a commission whose efforts to unify weights and measures led to the adoption of the metric system. Early 1788 Lavoisier became fellow of the Royal Society of London.
After the revolution of 1789, Lavoisier saw the change in power as an opportunity to rationalize and improve the nation's politics and economy. Being a child of the Enlightenment, he valued highly the power of reason professed by the revolutionary governments. He continued to advise them on finance and science matters, and neither he nor his wife fled abroad when Maximilien Robespierre became the de facto dictator of France and the reign of terror started. Lavoisier and his father-in-law were arrested on 28 November 1793; the Republic was being purged of its royalist past. In May 1794 Lavoisier, his father-in-law, and 26 other Tax Farmers were decapitated. Acknowledging Lavoisier's scientific stature, his contemporary, the great mathematician Joseph-Louis Lagrange, commented, "It took them only an instant to cut off that head, and a hundred years may not produce another like it."
Works[edit]
In the history of chemistry Lavoisier figures as the leader of the 18th-century chemical revolution and one of the founders of modern chemistry. Wealthy, ambitious, and goal-oriented, Lavoisier was the personification of Enlightenment rationality. He was a skillful investigator, but his main strength was quantification, interpretation, and demonstration. He was less of a discoverer than, for instance, Joseph Black, Henry Cavendish, and Joseph Priestley, to name a few other important 18th century chemists. Lavoisier's main goal was to position chemistry next to physics as a rigorous explanatory science.
Lavoisier's most important chemical investigations were made before the French Revolution of 1789. By 1785 Lavoisier's theory of combustion was gaining support, and his campaign started that was to put chemistry on the map as a scientific discipline. A definite step forward was publication (1789) of Traité élémentaire de chimie (Elementary Treatise on Chemistry). This treatise is generally seen as the first textbook on chemistry; it describes the precise methods chemists should employ when investigating, organizing, and explaining their subjects. In the book Lavoisier propagates a new nomenclature of chemistry that he had developed two years earlier with three of his prominent colleagues, among whom Claude Louis Berthollet. Much of this nomenclature is still in use today.
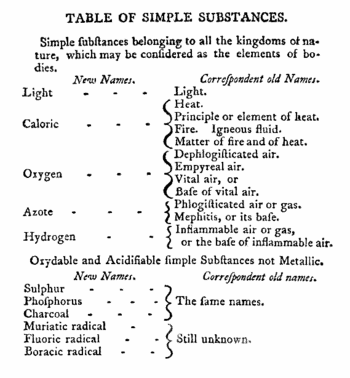
Chemistry of gases[edit]
In the beginning of the 18th century, many natural philosophers still viewed the four elements of Aristotle—Earth, Air, Fire, and Water—as the primary constituents of all matter, even though Robert Boyle had earlier expressed considerable doubts about it. There was already a considerable body of information (largely due to the alchemists) about chemical reactions, yet little agreement existed on the precise composition of chemical compounds. In those days it was widely believed that the Aristotelian elements could be distinguished by certain physical properties: water and earth were incompressible, air could be both expanded and compressed, whereas fire could not be contained or measured.
Around 1730 the English clergyman and natural philosopher Stephen Hales, whose main interest in "pneumatic chemistry" (chemistry of gases) was the development of plant life, demonstrated that air can be "fixed" in solids and liquids. Hales suggested that air was really just a vapor, like steam. Hales's experiments were an important first step in the experimental study of the chemistry of gases (or "airs" as they were then called by many, although Lavoisier himself preferred "gaz". In 1772 he used the word gaz for "fixed air" and from 1774 onward he used the term for different species of "air").
In the 1750s, the medical man Joseph Black demonstrated experimentally that air fixed in certain reactions is chemically different from atmospheric air. At the time that Black undertook his investigations, it was known that "mild alkalis" combine with phlogiston (i.e., lose oxygen) to became "caustic alkalis". For instance, limestone (calcium carbonate) becomes quicklime (calcium oxide) when heated. Black investigated the reverse reaction: the caustic quicklime is neutralized when exposed to the atmosphere. He found that quicklime absorbs only one component of the atmosphere, namely carbon dioxide (CO2), which he called "fixed air." Black's work marked the beginning of pneumatic chemistry, an area of research that grew rapidly during the latter half of the 18th century. In Lavoisier's times there were known: "residual air" or "dead air" (N2), "nitrous air" (NO), "inflammable air" (H2), "fixed air" (CO2), and "pure air", also known as "dephlogisticated air" or "fiery air" (O2).
Thus, the chemistry of gases was a lively subject when Lavoisier became interested in the related phenomena of combustion, respiration, and what 18th-century chemists called calcination (the change of metals to a powder [calx], such as that obtained by the rusting of iron when exposed to air). Lavoisier focused his attention upon analyzing compounds, such as the salts formed when acids combine with alkalis, using the balance as his main instrument. He hoped that by carefully weighing simple substances before and after reacting, it would become possible to construct chemical theories.
Oxygen versus phlogiston[edit]
Around 1715 the German chemist Georg Ernst Stahl hypothesized that a "fiery substance", which he called phlogiston (from φλοξ, flame), was released during combustion, respiration, and calcination. These are reactions (now called oxidation processes) in which, according to modern chemistry, oxygen is absorbed. In addition, Stahl proposed that phlogiston was absorbed when these processes were reversed. As is known now, oxygen is released in these reverse (reduction) reactions. Thus, phlogiston can be seen as a kind of "anti-oxygen". At the time that Lavoisier initiated his research, he accepted the existence of phlogiston as a working hypothesis.
The phlogiston theory did not hold very long, the discovery in 1774 by Priestley of oxygen (O2) tolled its death knell. The theory was finally replaced by Lavoisier's oxygen theory that resulted from an exhaustive set of experiments, performed from the early 1770s until 1783, aimed at an explanation of oxidation processes. The last important piece of the puzzle fell into place when Lavoisier heard in June 1783 from Sir Charles Blagden, a mutual friend of Cavendish and Lavoisier, that Cavendish had shown that water, and water only, appeared in the combination of "inflammable air" (H2) and "dephlogisticated air" (O2). Lavoisier interpreted this correctly as the oxidization of hydrogen by oxygen, confirmed the experiment in his laboratory, [2] and presented the result on November 12th, 1783 to the Paris Academy of sciences. In his announcement Lavoisier makes no reference to Cavendish as the discoverer. The priority of this discovery—the constitution of water, a milestone in the development of chemistry—gave rise to extensive controversy, with the final verdict that Cavendish discovered it and that Lavoisier was the first to give its correct interpretation. In the same year (1783) Lavoisier opened the attack on the "followers of Stahl", the phlogistonists; earlier Lavoisier had not expressed himself on the issue of phlogiston.
Lavoisier's research, lasting more than a decade and leading to the rejection of phlogiston, focused initially (the early 1770s) upon weight gains and losses in calcination. It was known that when metals slowly change (oxidize) into powders (calxes), the calx actually weighed more than the original metal, whereas when the calx was reduced to a metal, a loss of weight occurred. The phlogiston theory did not account for these weight changes, because fire, from which phlogiston was believed to escape, could not be isolated and weighed. Lavoisier hypothesized that it was probably the fixation and release of an "air", rather than fire, that caused the observed gains and losses in weight. This idea set the program of his research for the next decade.
During his researches, he encountered related phenomena that had to be explained. Sulfuric acid, for instance, was made by roasting sulfur in fire and then mixing the resultant calx with water. Lavoisier had initially conjectured that the sulfur combined with an "air" in the fire and that this "air" was the cause of acidity. However, it was not at all obvious just what kind of gas (air) made sulfur acidic.
The problem was further complicated by the multitude of known "airs". Most of them were discovered in Britain, by Joseph Priestley and Henry Cavendish. It was Priestley, a phlogistonist, who gave an important hint to Lavoisier in his unraveling of the mystery of the oxidation processes. Priestley isolated oxygen in August 1774 by heating red mercury oxide then called mercury precipitate per se. In Paris at the same time, Lavoisier and his colleagues were performing very similar experiments, but they failed to notice the novel properties of the "air" they collected. Priestley visited Paris in October 1774 and informed his French colleagues about the properties of this newly discovered "air". Lavoisier, who held Priestley's researches in high regard, repeated the experiment, and found that it produced precisely the kind of "air" he needed to complete his theory.
Lavoisier called the gas that was produced "oxygen" (the generator of acidity), because he was in the mistaken belief that all acids contain oxygen (sulfuric and nitric acid indeed do, but hydrochloric acid—"spirits of salt"—does not). In his first publication on oxygen[3] (May 1775) Lavoisier does not mention Priestley, though in later writings he acknowledges his priority.
After it became possible to isolate oxygen, the road was opened to the explanation, both quantitatively and qualitatively, of the oxidation processes combustion, respiration, and calcination. As already mentioned, it culminated in the determination of the constitution of water.
Mass and heat[edit]
Like many of his contemporaries, Lavoisier believed that matter was neither created nor destroyed in chemical reactions, and in his experiments he sought to demonstrate that the total mass of the reactants was conserved. In his Traité élémentaire de chimie Lavoisier expresses clearly and unambiguously the law of mass conservation.
During Lavoisier's time there were two conflicting theories of heat. One view considered heat as an indestructible and massless fluid, called caloric, and the other theory saw it as a form of motion (i.e., as a form of kinetic energy). On the whole Lavoisier favored the caloric view, but he did not perceive the two theories as contradictory. For example, he wrote together with the mathematician Pierre-Simon Laplace in 1783:[4]
We will not decide at all between the two foregoing hypotheses; several phenomena seem favorable to the one such as the heat produced by the friction of two solid bodies, for example, but there are others which are explained more simply by the other—perhaps both hold at the same time [....]. In general, one can change the first hypothesis into the second by changing the words "free heat", "combined heat" and "heat released" into "life force", "loss of life force", and "increase of life force".
It should be noted that "life force", introduced in 1695 by Leibniz under the Latin name vis viva, is (almost) equal to what is now known as kinetic energy.
References[edit]
- ↑ 1.0 1.1 Antoine Lavoisier Traité élémentaire de chimie, 2 vols. Chez Cuchet, Paris (1789). Translated from the French by Robert Kerr, Elements of Chemistry, 4th edition. William Creech, Edinburgh: (1790) p. 175. Google books Vol 1, Google books Vol 2
- ↑ June 24, 1783: Experiments on the production of water by detonating oxygen and hydrogen under a bell jar, carried out in the presence of Blagden, Laplace, Vandermonde, Fourcroy, Meusnier, Legendre and Le Roy
- ↑ Mémoire sur la nature du principe qui se combine avec les métaux pendant leur calcination et qui en augmente le poids. (Memoir on the nature of the principle which combines with metals during their calcination and which augments their weight). Mémoires de l’Académie des sciences, année 1775. p. 520 Online
- ↑
Mémoire sur la Chaleur, par MM. Lavoisier et de Laplace, Mémoires de l’Académie des sciences, année 1780, p. 355. Original French text:
Nous ne déciderons point entre les deux hypothèses précédentes ; plusieurs phénomènes paraissent favorables à la dernière ; tel est, par exemple, celui de la chaleur que produit le frottement de deux corps solides ; mais il en est d’autres qui s’expliquent plus simplement dans la première ; peut-être ont-elles lieu toutes deux à la foi [...] En général, on fera rentrer la première hypothèse dans la seconde, en y changeant les mots de chaleur libre, chaleur combinée et chaleur dégagée, dans ceux de force vive, perte de force vive, et augmentation de force vive. Online pp. 286-287 This memoir is predated, it was read before the Academy on June 28, 1783.
External links[edit]
- Les Œuvres de Lavoisier Collected works
- Panopticon Lavoisier First entry gives detailed time line of Lavoisier's life
 KSF
KSF