Asthma
 From Citizendium - Reading time: 8 min
From Citizendium - Reading time: 8 min
Asthma is a chronic inflammatory disorder of the airways, closely associated with an overexpression of immunoglobulin E (IgE). [1] At the cellular level, it is associated with an inflammatory response including infiltration of cells including neutrophils, eosinophils and lymphocytes; mast cell activation; and injury to epithelial cells.
"Inflammation contributes to airway hyperresponsiveness, airflow limitation, respiratory symptoms, and disease chronicity. In some patients, persistent changes in airway structure occur, including sub-basement fibrosis, mucus hypersecretion, injury to epithelial cells, smooth muscle hypertrophy, and angiogenesis."
Atopy, or genetic predisposition to an IgE response to common allergens, is the strongest known predisposing factors. Viral respiratory infections exacerbate existing disease and may contribute to the development of asthma.
Epidemiology[edit]
Etiology[edit]
According to the USA's National Heart, Lung, and Blood Institute of the National Institutes of Health, "atopy, the genetic predisposition for the development of an immunoglobulin E (IgE)-mediated response to common aeroallergens, is the strongest identifiable predisposing factor for developing asthma".
Atopy, although genetically predetermined, is exacerbated by environmental factors. Diesel gas emissions were shown to increase atopy in asthma in the same way as allergen exposure itself. As was emphasised by the researchers who isolated this effect, the demonstration of such a modification in the expression of genetic predispositions, which was achieved through epigenetic mechanisms, urges researchers to adopt a "new paradigm" in asthma and atopy management.[2]
Pathophysiology[edit]
Diagnosis[edit]
Asthma may be overdiagnosed.[3]
Prognosis[edit]
Many patients do well in spite of stopping their medications.[4][5]
Clinical practice guidelines recommend stepping down treatment for patients with asthma after symptoms have been controlled for at least three months.[6][7]
Treatment[edit]
Treatment of acute exacerbations[edit]
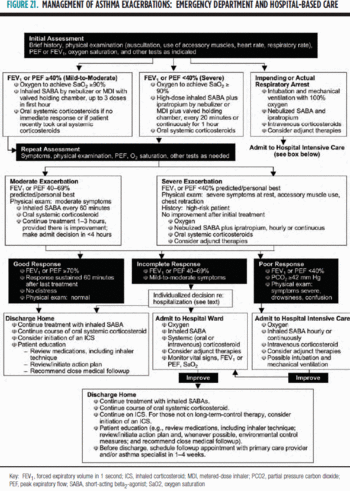
Classification[edit]
The U.S. National Asthma Education and Prevention Program defines exacerbations as:[8]
- Mild. "Dyspnea only with activity (assess tachypnea in young children)"; peak expiratory flow rate ≥70 percent predicted or personal best
- Moderate. "Dyspnea interferes with or limits usual activity"; peak expiratory flow rate 40−69 percent predicted or personal best
- Severe. "Dyspnea at rest; interferes with conversation"; peak expiratory flow rate <40 percent predicted or personal best
- Life threatening. "Too dyspneic to speak; perspiring"; peak expiratory flow rate <25 percent predicted or personal best
Drug therapy[edit]
Beta-adrenergic agonists[edit]
Albuterol, a beta-adrenergic agonists may be given 0.5 mg/kg/hour by continuous nebulization, although this may not reduce rates of hospitalization[9]. In children albuteral may be given as 0.3 mg/kg/hour by continuous nebulization.[10]
As opposed to short-acting beta-adrenergic blockers such as albuterol, which have both prophylactic and treatment roles, the long-acting variants, such as salmeterol, have no place in an acute attack.
Beta-adrenergic agonists[edit]
It is not clear whether adding ipratropium helps.[11]
Corticosteroids[edit]
Regarding corticosteroids, a systematic review concluded:[12]
- "There is no evidence that corticosteroid doses greater than standard doses (prednisone 50-100 mg equivalent) are beneficial. Oral and intravenous corticosteroids, as well as intramuscular and oral corticosteroid regimens, seem to be similarly effective. A nontapered 5- to 10-day course of corticosteroid therapy seems to be sufficient for most discharged patients. Combinations of oral and inhaled corticosteroids on emergency department/hospital discharge might minimize the risk of relapse."
At the first signs of an attack, quadrupling[13] but not doubling[14], the dose of inhaled corticosteroid may reduce the risk of an exacerbation of asthma requiring treatment with oral corticosteroids.
Patients that have been prescribed inhalers that combine a corticosteroid with a long-acting beta-adrenergic blocker, however, must understand that they cannot increase the use of that inhaler to get more corticosteroid. To do so could give a dangerous overdose of the beta-agonist.
Leukotriene antagonists[edit]
Leukotriene antagonists, montelukast or zafirlukast, may help.
Magnesium[edit]
Magnesium sulfate 2 grams in 50 mL of normal saline intranvenously over 15 minutes may help.[15]
Chronic treatment[edit]
Chronic asthma is an inflammatory disease. The core of modern treatment is preventing the inflammation, and using bronchodilators only when the inflammation goes out of control and causes bronchospasm.
Drug therapy[edit]
Many drugs beneficial for managing asthma are need to be inhaled into the bronchi and lungs. The simplest device for administering them is a metered-dose inhaler (MDI), which, in its basic form, produces a puff of aerosol to be inhaled with a single breath. Unfortunately, it takes a certain amount of dexterity to actuate the inhaler and take a simultaneous breath. By inserting a spacer chamber between the MDI and the mouthpiece, so the dose can be taken with several nonsynchronized breaths, administration becomes much more reliable. Spacers are a standard of care for children and animals, unless a nebulizer is used to deliver an aerosol using a powered pump that takes no special effort of inhalation.
Beta-adrenergic agonists[edit]
These are bronchodilators. They may be administered in metered-dose inhaler, nebulizer, or oral and parenteral forms.
Short acting beta-agonist agents are the initial treatment.
Long-acting adrenergic beta-agonists may help[16]; however, they should not be used without corticosteroids and maybe should not be used in African American patients.[17] As opposed to short-acting beta-adrenergic blockers such as albuterol, which have both prophylactic and treatment roles, the long-acting variants, such as salmeterol, have no place in an acute attack but may provide better overall control. Nevertheless, a higher death rate has been associated with the long-acting drugs.
They might be safe in asthma as long as corticosteroids are used; however they should be stopped if possible once asthma is controlled[18]. Single-nucleotide polymorphisms of the ADBR2 subtype of adrenergic receptor may affect response to adrenergic beta-agonists. According to a meta-analyses by the Cochrane Collaboration, when used with corticosteroids the relative risk for asthma-related death is increased at 1.34 although this increase was not statistically significant with a confidence interval of 0.30 to 5.97.[16][19] One meta-analysis found increased death even if corticosteroids are used; however, this meta-analysis excluded trials in which no deaths or intubations occurred.[20] A meta-analysis found not increased risk among patients taking salmeterol and corticosteroids.[21]
Corticosteroids[edit]
Inhaled corticosteroids have little if any systemic absorption, and have become a mainstay of the antiinflammatory component of asthma management.
Oral corticosteroids may be necessary in the management of severe asthma, but their many side effects offset their undoubted value. They are, however, fairly safe when used in short courses for exacerbations. For more severe disease, a specialist should be involved in finding ways to minimize their dose and adverse effects. These include such things as alternate-day dosing, or combining them with low-dosages of antimetabolites such as methotrexate.
Intravenous corticosteroids are part of the emergency treatment of asthma, along with nebulized bronchodilators. The intravenous route does not provide an appreciably faster onset of action than oral administration, but, of course, can be given to a patient who cannot swallow.
Methylxanthines[edit]
Theophylline is an orally administered bronchodilator, generally relegated to third-line status. It has a narrow therapeutic index and many drug interactions, usually because one or the other up- or down-regulates a Cytochrome P450 excretory pathway. Especially when used with other drugs, frequent blood level monitoring is wise to avoid toxicity.
Mast cell stabilizers[edit]
Leukotriene antagonists[edit]
Leukotriene antagonists may help.[22]
Environmental management[edit]
High-efficiency particulate air (HEPA) cleaners may help.[23]
Monitoring[edit]
A systematic review by the Cochrane Collaboration found that monitoring sputum eosinophils can guide treatment[24] The review identified three randomized controlled trials that found that benefit from adjusting anti-inflammatory medications to maintain less than 2 to 8% eosinophils in sputum.
Regarding peak expiratory flow rate monitoring, according to a meta-analysis of randomized controlled trials by the Cochrane Collaboration, peak flow monitoring is equivalent to symptom monitoring.[25] The U.S. National Asthma Education and Prevention Program recommends peak expiratory flow rate monitoring for selected patients.[8] A more recent trial, with improved methods of symptom monitoring and education, found that peak flow monitoring did not improvement asthma control.[26]
More recently, studies have examined graphical display of peak flow monitoring and reported improved outcomes by increasing routing use of inhaled corticosteroids[27] and in a second study, no benefit[28] in information.
References[edit]
- ↑ National Asthma Education and Prevention Program (2002), Section 2, Definition, Pathophysiology and Pathogenesis of Asthma, and Natural History of Asthma, Expert Panel Report 2: Guidelines for the diagnosis and management of asthma., National Institutes of Health
- ↑ Liu J, Ballaney M, Al-alem U, et al (March 2008). "Combined inhaled diesel exhaust particles and allergen exposure alter methylation of T helper genes and IgE production in vivo". Toxicol. Sci. 102 (1): 76-81. DOI:10.1093/toxsci/kfm290. PMID 18042818. Research Blogging.
- ↑ Aaron, Shawn D.; Katherine L. Vandemheen, Louis-Philippe Boulet, R. Andrew McIvor, J. Mark FitzGerald, Paul Hernandez, Catherine Lemiere, Sat Sharma, Stephen K. Field, Gonzalo G. Alvarez, Robert E. Dales, Steve Doucette, Dean Fergusson, for the Canadian Respiratory Clinical Research Consortium (2008-11-18). "Overdiagnosis of asthma in obese and nonobese adults". CMAJ 179 (11): 1121-1131. DOI:10.1503/cmaj.081332. Retrieved on 2008-11-25. Research Blogging.
- ↑ Cramer JA, Bradley-Kennedy C, Scalera A (2007). "Treatment persistence and compliance with medications for chronic obstructive pulmonary disease.". Can Respir J 14 (1): 25-9. PMID 17315055. PMC PMC2690446. [e]
- ↑ Kang MG, Kim JY, Jung JW, Song WJ, Cho SH, Min KU et al. (2013). "Lost to follow-up in asthmatics does not mean treatment failure: causes and clinical outcomes of non-adherence to outpatient treatment in adult asthma.". Allergy Asthma Immunol Res 5 (6): 357-64. DOI:10.4168/aair.2013.5.6.357. PMID 24179681. PMC PMC3810541. Research Blogging.
- ↑ Global Initiative for Asthma (GINA). Global strategy for asthma management and prevention. Vancouver (WA): Global Initiative for Asthma (GINA); 2012. 110 p.
- ↑ Management of Asthma Working Group. VA/DoD clinical practice guideline for management of asthma in children and adults. Washington (DC): Department of Veteran Affairs, Department of Defense; 2009. 126 p.
- ↑ 8.0 8.1 National Asthma Education and Prevention Program: Expert Panel Report III: Guidelines for the diagnosis and management of asthma. Bethesda, MD. National Heart, Lung, and Blood Institute, 2007. (NIH publication no. 08-4051). Available from http://www.nhlbi.nih.gov/guidelines/asthma/asthgdln.htm. (Accessed September 1, 2008).
- ↑ Rodrigo GJ, Rodrigo C (2002). "Continuous vs intermittent beta-agonists in the treatment of acute adult asthma: a systematic review with meta-analysis.". Chest 122 (1): 160-5. PMID 12114352.
- ↑ Papo MC, Frank J, Thompson AE (1993). "A prospective, randomized study of continuous versus intermittent nebulized albuterol for severe status asthmaticus in children.". Crit Care Med 21 (10): 1479-86. PMID 8403956.
- ↑ Salo D, Tuel M, Lavery RF, Reischel U, Lebowitz J, Moore T (2006). "A randomized, clinical trial comparing the efficacy of continuous nebulized albuterol (15 mg) versus continuous nebulized albuterol (15 mg) plus ipratropium bromide (2 mg) for the treatment of acute asthma.". J Emerg Med 31 (4): 371-6. DOI:10.1016/j.jemermed.2006.05.025. PMID 17046476. Research Blogging.
- ↑ Krishnan JA, Davis SQ, Naureckas ET, Gibson P, Rowe BH (2009). "An umbrella review: corticosteroid therapy for adults with acute asthma.". Am J Med 122 (11): 977-91. DOI:10.1016/j.amjmed.2009.02.013. PMID 19854321. PMC PMC2768615. Research Blogging.
- ↑ Oborne J, Mortimer K, Hubbard RB, Tattersfield AE, Harrison TW (2009). "Quadrupling the dose of inhaled corticosteroid to prevent asthma exacerbations: a randomized, double-blind, placebo-controlled, parallel-group clinical trial.". Am J Respir Crit Care Med 180 (7): 598-602. DOI:10.1164/rccm.200904-0616OC. PMID 19590019. Research Blogging.
- ↑ Harrison TW, Oborne J, Newton S, Tattersfield AE (2004). "Doubling the dose of inhaled corticosteroid to prevent asthma exacerbations: randomised controlled trial.". Lancet 363 (9405): 271-5. PMID 14751699. Review in: ACP J Club. 2004 Sep-Oct;141(2):37
- ↑ Silverman RA, Osborn H, Runge J, Gallagher EJ, Chiang W, Feldman J et al. (2002). "IV magnesium sulfate in the treatment of acute severe asthma: a multicenter randomized controlled trial.". Chest 122 (2): 489-97. PMID 12171821.
- ↑ 16.0 16.1 Walters EH, Gibson PG, Lasserson TJ, Walters JA (2007). "Long-acting beta2-agonists for chronic asthma in adults and children where background therapy contains varied or no inhaled corticosteroid". Cochrane Database Syst Rev (1): CD001385. DOI:10.1002/14651858.CD001385.pub2. PMID 17253458. Research Blogging.
- ↑ Salpeter SR, Buckley NS, Ormiston TM, Salpeter EE (2006). "Meta-analysis: effect of long-acting beta-agonists on severe asthma exacerbations and asthma-related deaths". Ann. Intern. Med. 144 (12): 904-12. PMID 16754916. [e]
- ↑ Chowdhury BA, Dal Pan G (2010). "The FDA and Safe Use of Long-Acting Beta-Agonists in the Treatment of Asthma.". N Engl J Med. DOI:10.1056/NEJMp1002074. PMID 20181964. Research Blogging.
- ↑ Cates CJ, Cates MJ (2008). "Regular treatment with salmeterol for chronic asthma: serious adverse events". Cochrane Database Syst Rev (3): CD006363. DOI:10.1002/14651858.CD006363.pub2. PMID 18646149. Research Blogging.
- ↑ Salpeter SR, Wall AJ, Buckley NS (2010). "Long-acting beta-agonists with and without inhaled corticosteroids and catastrophic asthma events.". Am J Med 123 (4): 322-8.e2. DOI:10.1016/j.amjmed.2009.07.035. PMID 20176343. Research Blogging.
- ↑ Bateman E, Nelson H, Bousquet J, Kral K, Sutton L, Ortega H et al. (2008). "Meta-analysis: effects of adding salmeterol to inhaled corticosteroids on serious asthma-related events.". Ann Intern Med 149 (1): 33-42. PMID 18523132.
- ↑ Price D, Musgrave SD, Shepstone L, Hillyer EV, Sims EJ, Gilbert RF et al. (2011). "Leukotriene antagonists as first-line or add-on asthma-controller therapy.". N Engl J Med 364 (18): 1695-707. DOI:10.1056/NEJMoa1010846. PMID 21542741. Research Blogging.
- ↑ Butz AM, Matsui EC, Breysse P, Curtin-Brosnan J, Eggleston P, Diette G et al. (2011). "A randomized trial of air cleaners and a health coach to improve indoor air quality for inner-city children with asthma and secondhand smoke exposure.". Arch Pediatr Adolesc Med 165 (8): 741-8. DOI:10.1001/archpediatrics.2011.111. PMID 21810636. Research Blogging.
- ↑ Petsky H, Kynaston J, Turner C, et al (2007). "Tailored interventions based on sputum eosinophils versus clinical symptoms for asthma in children and adults". Cochrane database of systematic reviews (Online) (2): CD005603. DOI:10.1002/14651858.CD005603.pub2. PMID 17443604. PMID 17443604. Research Blogging.
- ↑ Powell H, Gibson PG (2003). "Options for self-management education for adults with asthma". Cochrane Database Syst Rev (1): CD004107. PMID 12535511. [e]
- ↑ Buist AS, Vollmer WM, Wilson SR, Frazier EA, Hayward AD (2006). "A randomized clinical trial of peak flow versus symptom monitoring in older adults with asthma.". Am J Respir Crit Care Med 174 (10): 1077-87. DOI:10.1164/rccm.200510-1606OC. PMID 16931634. PMC PMC2648108. Research Blogging. Review in: Evid Based Nurs. 2007 Apr;10(2):52 Review in: Evid Based Med. 2007 Apr;12(2):49
- ↑ Janson SL, McGrath KW, Covington JK, Baron RB, Lazarus SC (2010). "Objective airway monitoring improves asthma control in the cold and flu season: a cluster randomized trial.". Chest 138 (5): 1148-55. DOI:10.1378/chest.09-2394. PMID 20538819. PMC PMC2972622. Research Blogging.
- ↑ Turner RM, Hayen A, Macaskill P, Irwig L, Reddel HK (2012). "Control charts demonstrated limited utility for the monitoring of lung function in asthma.". J Clin Epidemiol 65 (1): 53-61. DOI:10.1016/j.jclinepi.2011.04.012. PMID 21803547. Research Blogging.
 KSF
KSF