Borna disease virus
 From Citizendium - Reading time: 14 min
From Citizendium - Reading time: 14 min
| Borna disease virus | ||||||
|---|---|---|---|---|---|---|
 | ||||||
| Virus classification | ||||||
|
Classification[edit]
ICTVdB Virus Code: 01.081.0.01.001. Virus accession number: 81001001. Obsolete virus code: 81.0.1.0.001; superseded accession number: 81010001. NCBI Taxon Identifier NCBI Taxonomy ID: 12455. Type of the genus: 01.081.0.01 Bornavirus; Family: 01.081 Bornaviridae; Order: 01 Mononegavirales.[1]
Viruses: Group V ssRNA viruses; Order: Mononegavirales; Family: Bornaviridae; Genus: Bornavirus
Description and significance[edit]
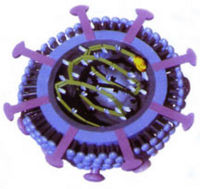
A nonsegmented, negative, single-stranded, enveloped RNA virus that is spherical in shape with a total size of 80-125 nm.[2] Its core is 50-60 nm in diameter, and its envelope has surface projections approximately 7 nm long that evenly cover the surface.[1]
It is neurotropic and noncytolytic and has a wide geographic distribution and host range,[3] including horses, sheep, and humans. A growing body of literature links Borna disease virus (BDV) infections to a range of neurological disorders often characterized by manic-depressive symptoms. Some studies have demonstrated a therapeutic effect of the antiviral agents amantadine and ribavirin in BDV-infected, depressed patients.
BDV research has implications on a wide range of fields from molecular biology and immunology to psychiatry, criminal justice, and education.
Natural Hosts[edit]
Domain Eucarya, Kingdom Animalia, Phylum Chordata, Subphylum Vertebrata, Class Mammalia and Aves.
Class Aves, Order Struthioniformes, Family Struthionida (ostrich).
Class Mammalia, Order Scandentia, Family Tupaiidae, Genus Tupaia; Order Primates, Family Hominidae, Genus Homo, Species H. sapiens (human); Order Carnivora, Suborder Fissipedia, Family Felidae, Subfamily Felinae, Genus Felis (cats); Order Perissodactyla, Family Equidae, Genus Equus, Species E. caballus (horse); Order Artiodactyla, Family Bovidae, Subfamily Bovinae, Genus Bos, Species B. taurus (cow); Order Artiodactyla, Family Bovidae, Subfamily Caprinae, Genus Ovis, Species O. aries (sheep); Order Rodentia, Suborder Scurognathi, Family Muridae, Subfamily Murinae, Genus Rattus (rat).[1]
Discovery[edit]
Borna disease was first discovered in horses in Borna, Saxony, Germany in 1763[4] although descriptions of the disease can be traced back to 1660.[5] Borna disease virus was characterized as the causative agent in the early 1900s by Zwick and co-workers in Gieseen, Germany.[3]
Isolation[edit]
Most experimental studies on the virus use BDV isolates obtained from the brains of rats, though serological evidence for a link between BDV and neurobehavioral deficits in humans is typically obtained by examining human blood samples for the presence of BDV RNA, p40, or p24 protein.
The first cDNA clones of the BDV genome were isolated in the early 1990s by a number of different research groups. In 1994, Cubitt and colleagues isolated BDV RNA from the C6 cells of experimentally infected rats at The Scripps Research Institute in La Jolla, California prior to sequencing.[6] In that year, Briese and coworkers at the University of California, Irvine similarly obtained clones from rat oligodendrocyte isolates.[7] The sequence analyses were performed using BDV specific cDNA probes.
Genome structure[edit]
BDV has a ca. 8.9 kb genome size of encapsulated, non-segmented, single-stranded RNA[3][8] with six open reading frames (ORFs). ORFs I-V correspond to a nucleoprotein 40-kDa p40 (N); a phosphoprotein 23-kDa p23 (P); a matrix protein 16-kDa p16 (M); a glycoprotein 57-kDa p57 (G); and an L-polymerase 190-kDa p190 (L), respectively.[7] A sixth protein, p10 (X), likely functions to inhibit apoptosis and promote persistence in the central nervous system (CNS).[9] The ORFs are flanked by 53 nt of noncoding sequence at the 3' terminus and 91 nt of noncoding sequence at the 5' terminus.[7]
The BDV genome is homologous to the other Mononegavirales in at least five (I-V) of the ORFs.[7]
Interesting features[edit]
The BDV genome is smaller than that of other negative-strand RNA viruses. Unlike other non-segmented, negative-strand RNA animal viruses and Mononegavirales, BDV replicates and transcribes in the nuclei of its hosts. It has a number of other features that distinguish it from all other Mononegavirales, such as overlap of coding sequence, post-transcriptional modification properties, and alternative splicing in the nuclei of its host.[7] It is also highly unusual in certain aspects of its pathogenesis, including its highly specific localization in the CNS, manipulation of activity-dependent synaptic plasticity, and its chronic persistence in the absence of overt inflammation and cytolysis.[10] As a whole, the BDV genome has a remarkable degree of conservation with less than 6% divergence at the nucleotide level.[11]
Pathogenesis[edit]
The pathogenesis of Borna disease is a subject of intense debate.
BDV is assumed to be transmitted through nasal, salival, or conjunctival secretions. It likely gains access to the CNS via intraaxonal migration through the olfactory nerve or nerve endings in the oropharyngeal and intestinal regions. The virus spreads throughout the CNS by intraaxonal transport and centrifugally into the peripheral nerves.[3] It gains access to target cells via receptor-mediated endocytosis in a process facilitated by the viral surface glycoprotein.[12]
Some studies have shown that the disease is at least partially mediated by immune system.[13] Such models are supported by evidence that immunocompromised Lewis rats do not become ill even when infected with BDV. Several of these reports suggest that BDV-altered T cell responses contribute to the pathogenesis.[14][15]
More recent reports have largely widened the realm of etiological suspects, implicating BDV-mediated effects on synaptic plasticity,[16] PKC pathways,[10] and the activation of microglia.[17]
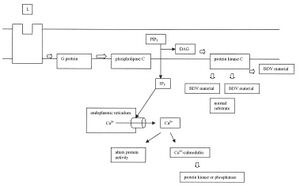
-The diagram below outlines the effect of BDV on the PKC pathway.
Here we see that BDV or its proteins have an effect downstream of PKC activation. It disrupts the pathway by competing with the normal substrates of PKC.
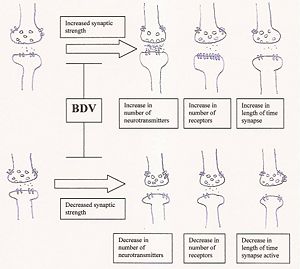
-The following image diagrams the effect of BDV infection on synaptic plasticity.
Synaptic plasticity is defined as the ability to increase or decrease synaptic strength, and synaptic strength can be altered by modifying the number of neurotransmitters released; the number of postsynaptic receptors; or the reuptake of neurotransmitters by the presynaptic neuron, altering the length of time that the signal is active. BDV infection is purported to impair the ability to modulate such alterations in synaptic strength.
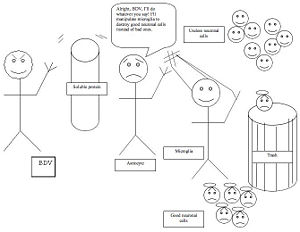
-The pathogenetic mechanism for BDV activation of microglia is demonstrated in the image below.
Here, Borna disease virus sends out soluble protein factors in order to induce astrocytes to promote the activation of microglia. Microglia, which normally serve a useful function in ridding the brain of unwanted neurons, is then induced to destroy normal neurons in the dentate gyrus of the hippocampus.
What makes it biologically interesting?[edit]
- Several reports have indicated that BDV infection may cause obesity in Lewis rats. BDV infection may stimulate obesity through inflammation and an increase in viral proteins in the hypothalamus, an area of the brain that is often involved in weight gain.[18]
- Some studies have found Ara-C (1-beta-arabinofuranosylcytosine), a drug often used in chemotherapy, to be effective in inhibiting the replication and spread of BDV.[19]
- Many studies have examined the effectiveness of amantadine against BDV. Amantadine was approved by the US Food and Drug Administration for use as a prophylactic against influenza in the 1960s and was shortly thereafter found to reduce symptoms of Parkinson's disease. There is debate among the scientific community as to the usefulness of amantadine as a therapeutic drug in reducing infection and psychiatric symptoms among BDV-infected patients with affective disorders. Some researchers have found evidence that amantadine significantly reduces mania and depression in these patients.[20][21][13][22] Other studies found no antiviral activity of amantidine on BDV.[23][24]
Current Research[edit]
Reports on the link between Borna disease virus and neurological deficits.
Antibodies to Borna disease virus in subacute sclerosing panencephalitis.[25] This report sought to determine whether children with subacute sclerosing panencephalitis (SSPE) were more likely than than children with noninfectious neurological disorders to test positive for the seroprevalence of Borna disease virus (BDV). SSPE is caused by persistent infection of immune-resistant measles virus. BDV shares a wide variety of features with the measles virus, so the authors were interested in examining what links, if any, might exist between BDV and SSPE. They collected blood samples from 174 children with SSPE and from 173 children with nonprogressive, noninfectious neurological disorders (epilepsy, headache, cerebral palsy) from the same geographical area and same rural vs. urban demographics. They used antibodies to BDV protein p40 as a test of seroprevalence and performed inter- and intra-group comparisons on a number of different variables. They found that SSPE children and their counterparts with noninfectious, neurological disorders were equally likely to test positive for BDV. But they found that SSPE children with high levels of BDV were significantly more likely to have earlier onset and a more rapid clinical course of the disease. The authors concluded that BDV may not be associated with the prevalence of SSPE but rather may contribute to many of its debilitating symptoms post-infection with measles.
Borna disease virus and mental health: a cross-sectional study.[26] This study examined the possible link between the prevalence of BDV in the blood and psychiatric morbidity among farmers. Because farmers and their families are known to be at higher risk for suicide, the study sought to determine if the interaction of farmers with animals suspected to be carriers of BDV was an explanatory factor. The authors randomly selected a group of subjects from arable, mixed, and livestock farming communities, analyzed their blood for BDV seroprevalence using a BDV enzyme-linked immuno-absorbance assay (ELISA), and conducted the Revised Clinical Interview Schedule (CIS-R) to test for neurobehavioral pathology. They found a higher seroprevalence among livestock farmers than mixed and arable farmers, but the results were not significant. Interestingly, farmers working with horses, cats, and most other animals had lower levels of seroprevalence. The researchers speculate that if psychiatric morbidity among farmers is, in fact, mediated by BDV infections, there may be a period of latency before psychiatric conditions develop. It is also possible that farmers with BDV-related psychiatric illness were less likely to participate in the study. Nevertheless, the results do not support a link between BDV exposure and psychiatric morbidity.
RNA from Borna disease virus in patients with schizophrenia, schizoaffective patients, and in their biological relatives.[5] This paper investigated Borna disease virus (BDV) p24 RNA prevalence in the blood of psychiatric patients, their biological relatives, and healthy controls. The authors extracted the total RNA from peripheral white blood cells and used RT-nested PCR for amplification of the BDV p24. They sequenced the products and then analyzed the results using information from the National Center for Biotechnology Information (NCBI) NIH database. The BDV p24 was found in 44.4% of patients with schizophrenia or schizoaffective disorder, 50.0% of their biological relatives without mental disorders, 37.5% of their biological relatives with mood disorders, and 14.8% of healthy controls. Patients and their biological relatives without mental disorders were found to be significantly more likely than healthy controls to test positive for the presence of BDV p24 RNA. The results add to a body of literature that suggests a link between BDV infection and neurobehavioral disorders, although the authors contend that BDV is likely only one among many interacting factors that may contribute to an individual's cumulative psychiatric status.
Reports on the pathogenesis of Borna disease virus
Borna disease virus infection impairs synaptic plasticity.[16] This paper examines the mechanisms through which Borna disease virus (BDV) causes neurobehavioral disorders. The authors took advantage of the newly designed multielectrode arrays (MEA), which consists of a grid of 60 planar electrodes embedded in a culture dish and concentrated on a 1mm square area. They grew both BDV-infected and control neurons in the sterile MEA chamber and stimulated them with bicuculline, a gamma-aminobutyric acid A receptor antagonist that has been shown to result in an increase in synaptic activity at excitatory synapses. Measuring electrophysiological responses in real time, the authors found that BDV has no impact on spontaneous neuronal activity or on the ability of the neurons to modulate synaptic transmission during stimulation. However, the researchers found significant differences between experimental and control neurons 1 hour post-stimulus. The control neurons maintained a high level of activity following bicuculline exposure 1 and 2 hours after stimulus, while the BDV-infected neurons had returned to basal levels. Because this post-stimulus activation increases the strength of synapatic connections and is implicated in processes associated with learning and memory, the results suggest that the neurobehavioral changes associated with BDV-infections may be a result of an impairment in synaptic plasticity.
Borna disease virus blocks potentiation of presynaptic activity through inhibition of protein kinase C signaling.[10] Since previous studies have demonstrated Borna disease virus (BDV)-infection to be associated with neurobehavioral disorders, the study investigated whether BDV has a deleterious effect on synaptic signaling. Protein kinases are known to have important roles in the early stages of synaptic signaling, so the researchers used Western blot analyses with phospho-specific antibodies to examine whether BDV-infections were associated with impairments in the functioning of extracellular-regulated kinase (ERK) 1/2, protein kinase A (PKA), calcium/calmodulin dependent kinase (CaMK) II, and PKC phosphorylate presynaptic proteins that modulate synaptic vesicle recycling. The researchers found no effect of BDV-infection on ERK 1/2, PKA, and CaMK II, but the phosphorylation of two major PKC substrates, myristoylated alanine-rich C kinase substrate (MARCKS) and Munc18-1/nSec1 (Munch18), was severely inhibited in BDV-infected neurons, indicating BDV interference with PKC signaling.
The authors followed with a number of related findings that shed light on the mechanisms whereby BDV might have an effect on the PKC signaling pathway. They discovered that BDV had no affect on the physical translocation of several different forms of PKC from the cytosol to membranes. Since this process plays a major role in the activation of PKC, the authors concluded that BDV-mediated effects on PKC signaling take place downstream of PKC activation. In another experiment, the authors also used vectors to express BDV phosphoprotein (BDV P) in non-infected neuronal tissue. They found that simply the expression of BDV P was sufficient to significantly decrease the phosphorylation of PKC substrates, suggesting that BDV's inhibition of PKC signaling may involve a mechanism whereby its phosphoprotein competes with the substrates of PKC for phosphorylation. Lastly, the study found that similar effects of PKA agonist forskolin on infected and control neurons, confirming that it is the PKC and not the cAMP/PKA pathway that is affected by BDV.
Proteomic analysis reveals selective impediment of neuronal remodeling upon Borna disease virus infection.[27] This study set out to explore differences in the proteomes of neurons infected with Borna disease virus (BDV) and non-infected controls. Control and infected cerebral cortical neurons were generated from embryonic Sprague-Dawley rats, fractionated with two-dimensional liquid chromatography (2D-LC), and their respective proteins identified and analyzed using nano-liquid chromatography (nanoLC)-tandem mass spectrometry (MS/MS). The analyses revealed BDV impairment of proteins involved in synaptic activity (changes in B-FABP, differences for MARCKS and mUNC18, significant downregulation of GAP-43, significant decrease in synapsin 1 levels); cytoskeleton dynamics (differences in 10 of the 28 actin-binding proteins and 5 of the 13 microtubule-binding proteins that are known to be associated with the neuronal core); mRNA regulation (significant decrease in MeCP2 levels); and mRNA expression (acetylation of the Histone H2B protein). The lack of evidence for a change in gross neuronal morphology is consistent with previous studies, and the paper suggests that the BDV alteration of proteins involved in neuronal signaling represents an overall strategy for long term survival and propagation of the virus.
Persistent Borna disease virus (BDV) infection activates microglia prior to a detectable loss of granule cells in the hippocampus.[17] This study aimed to determine whether microglia activation occurred before or after BDV-induced death of neuronal cells. Microglia, a type of glial cells and the resident macrophages of the brain, are generally quite important in ridding the brain of neurons that are not useful, thereby making room for those neurons that are more important. However, they can also be manipulated by environmental factors such as viruses to destroy neurons that are, in fact, useful. Microglia activation was known to be associated with BDV-induced neuron loss, but it wasn't clear whether microglia actually caused the neuronal cell death associated with BDV infection or whether they simply cleaned up dead cells that had already been killed by BDV through some other mechanism. The authors infected rats with BDV, sacrificed the animals at different time intervals after infection, measured neuronal cell number and mass, and used antibodies to ED1 as a specific marker of BDV-induced microglia activation. The authors found a significant in the levels of hippocampal ED1 prior to any significant loss of neuron cells or neuronal mass. The authors concluded that BDV may cause cell death in the hippocampus by inducing the activation of microglia.
Reports on the treatment of Borna disease virus infections
Ribavirin inhibits Borna disease virus proliferation and fatal neurological diseases in neonatally infected gerbils.[28] The report examined the effects of the antiviral drug ribavirin on microglia and Borna disease virus (BDV) RNA levels in BDV-infected gerbils, and they studied whether any antiviral effects of the drug were mediated by the immune system. The authors infected a group of gerbils with BDV, injected them with 0, 1 mg, 5 mg, and 10 mg doses of ribavirin, and perfused them at 25 days post-infection. They used semi-quantitative RT-PCR to determine the levels of BDV RNA for the nucleoprotein (N) region, and found that the gerbils treated with 10 mg ribavirin had significant reductions in BDV N mRNA for the cerebral cortex, hippocampus, lower brainstem, and cerebellum regions. Using an immunohistochemical analysis for OX-42 monoclonal antibody, they found a marked reduction of microglial activation in ribavirin-treated gerbils at 25 days post-infection. They also used semi-quantitative RT-PCR to determine whether certain Th1 cytokine levels were enhanced in the ribavirin groups but found no significant differences. The authors therefore concluded that ribavirin may well have effective antiviral properties in the treatment of BDV infections, but its effects are most likely at the level of BDV replication and do not involve an enhancement of Th1 immunity.
Human Borna disease virus-infection and its therapy in affective disorders[13] This paper reports on a number of studies by the authors and colleagues examining the antiviral effects of amantadine in BDV-infected human patients with affective disorders. In one small study with 25 subjects, the authors delivered amantadine to two groups of BDV-infected patients: those with a diagnosis of bipolar disorder or major depression without an axis-II diagnosis, and those with an axis-II diagnosis of a personality disorder. They found a significant reduction in depressive symptoms in the former but not the later group, and the clinical response was paralleled by a decrease in BDV-infection parameters. Another study with 16 subjects examined BDV-infected patients with an affective disorder. Half received amantadine and half received placebo. Curiously, both groups exhibited a reduction in BDV-parameters after 14 weeks. A smaller study with 10 subjects was carried out on BDV-infected bipolar patients who were recently severe hypomanic or moderately manic. The authors found a significant reduction in manic symptoms upon treatment with amantadine, but this was not paralleled by a reduction in BDV-parameters. Nevertheless, the results are impressive because the psychopharmacological properties of amantadine are such that it would be expected to increase mania. It is therefore intriguing that another effect of amantadine on this cohert is powerful enough to overcome and strongly reverse the inherent mania-promoting properties of this drug. The authors interpreted the findings as supporting the hypothesis that amantadine has significant therapeutic effects on BDV-infected patients with affective disorders.
References:[edit]
- ↑ 1.0 1.1 1.2 Buchen-Osmond, C. (Ed) (2003). 01.081.0.01.001. Borna disease virus. In: ICTVdB - The Universal Virus Database, version 3. Buchen-Osmond, C. (Ed), ICTVdB Management, Columbia University, New York, USA.
- ↑ Brooks, G.F., Butel, J.S., and Morse, S.A. (2001). Jawetz, Melnick, and Adelberg's Medical Microbiology, 22nd Edition. New York: McGraw-Hill. 320.
- ↑ 3.0 3.1 3.2 3.3 Richt, J.A., Pfeuffer, I., Christ, M., Frese, K., Bechter, K., Herzog, S. (1997). Borna disease virus infection in animals and humans. Emerging Infectious Diseases, 3(3). 343-352.
- ↑ Durrwald, L. (1997). Journal of Veterinary Medicine, 44. 147-184.
- ↑ 5.0 5.1 Nunes, S.O.V., Itano, E.N., Amarante, M.K., Reiche, E.M.V., Miranda, H.C., de Oliveira, C.E.C., Matsuo, T., Vargas, H.O., Watanabe, M.A.E. (2008). RNA from Borna disease virus in patients with schizophrenia, schizoaffective patients, and in their biological relatives. Journal of Clinical Laboratory Analysis, 22. 314-320.
- ↑ Cubitt, B., Oldstone, C., and de la Torre, J.C. (1994). Sequence and genome organization of Borna disease virus. Journal of Virology, 68(3). 1382-1396.
- ↑ 7.0 7.1 7.2 7.3 7.4 Briese, T., Schneemann, A., Lewis, A., Park, Y., Kim, H., Ludwig, H., and Lipkin, I. (1994). Genomic organization of Borna disease virus. Proceedings of the National Academy of Sciences, 91(10). 4362-66.
- ↑ de la Torre, J.C. (1994). Molecular biology of Borna Disease Virus: Prototype of a new group of animal viruses. Journal of Virology, 68(12). 7669-75.
- ↑ Poenisch, M., Burger, N., Staeheli, P., Bauer, G., and Schneider, U. (2009). Protein X of Borna disease virus inhibits apoptosis and promotes viral persistence in the central nervous systems of newborn-infected rats. Journal of Virology, 83(9). 4297-4307.
- ↑ 10.0 10.1 10.2 Volmer, R., Monnet, C., and Gonzalez-Dunia, D. (2006). Borna disease virus blocks potentiation of presynaptic activity through inhibition of protein kinase C signaling. PLoS Pathogens, 2(3). e19.
- ↑ Hornig, M., Briese, T., and Lipkin, W.I. (2003). Borna disease virus. Journal of NeuroVirology, 9. 259-79.
- ↑ Clemente, R., de Parseval, A., Perez, M., and de la Torre, J.C. (2009). Borna disease virus requires cholesterol in both cellular membrane and viral envelope for efficient cell entry. Journal of Virology, 83(6). 2655-2662.
- ↑ 13.0 13.1 13.2 Dietrich, D.E., and Bode, L. (2008). Human Borna disease virus-infection and its therapy in affective disorders. APMIS Supplement, 116. 61-65.
- ↑ Narayan, O., Herzog, S., Frese, K., Scheefers, H., and Rott, R. (1983). Behavioral disease in rats caused by immunopathological responses to persistent Borna visu in the brain. Science, 220(1). 401-3.
- ↑ Stitz, L., Dietzshold, B., and Carbone, K.M. (1995). Immunopathogenesis of Borna disease. Current Topics in Microbiology and Immunology, 190. 75-92.
- ↑ 16.0 16.1 Volmer, R., Prat, C.M.A., Le Masson, G., Garenne, A., and Gonzalez-Dunia, D. (2007). Borna disease virus infection impairs synaptic plasticity. Journal of Virology, 81(16). 8833-7.
- ↑ 17.0 17.1 Ovanesov, M.V., Moldovan, K., Smith, K., Vogel, M.W., and Pletnikov, M.V. (2008). Persistent Borna disease virus (BDV) infection activates microglia prior to detectable loss of granule cells in the hippocampus. Journal of Neuroinflammation, 5(16).
- ↑ Herden, C., Herzog, S., Richt, J.A., Nesseler, A., Christ, M., Failing, K., and Frese, K. (2000). Distribution of Borna disease virus in the brain of rats infected with an obesity-inducing virus strain. Brain Pathology, 10(1). 39-48.
- ↑ Bajramovic, J.J., Syan, S., Brahic, M., de la Torre, J.C., Gonzalez-Dunia, D. (2002). 1-beta-D-Arabinofuranosylcytosine inhibits Borna disease virus replication and spread. Journal of Virology, 76(12). 6268-6276.
- ↑ Bode, L., Dietrich, D.E., Stoyloff, R., Emrich, H.M., and Ludwig, H. (1997). Amantadine and human Borna disease virus in vitro and in vivo in an infected patient with bipolar depression. Lancet, 349(9056). 958.
- ↑ Dietrich, D.E., Bode, L., Spannhuth, C.W., Lau, T., Huber, T.J., Brodhun, B., Ludwig, H., and Emrich, H.M. (2000). Amantadine in depressive patients with Borna disease virus (BDV) infection: an open trial. Bipolar Disorder, 2(1). 65-70.
- ↑ Ohlmeier, M.D., Zhang, Y., Bode, L., Sieg, S., Feutl, S., Ludwig, H., Emrich, H.M., and Dietrich, D.E. (2008). Amantadine reduces mania in borna disease virus-infected non-psychotic bipolar patients. Pharmacopsychiatry, 41(5). 202-3.
- ↑ Cubitt, B., and de la Torre, J.C. (1997). Amantadine does not have antiviral activity against Borna disease. Archives of Virology, 142(10). 2035-42.
- ↑ Hallensleben, W., Zocher, M., and Staecheli, P. (1997). Borna disease virus is not sensitive to amantadine. Archives of Virology, 142(10). 2043-8.
- ↑ Gungor, S., Anlar, B., Turan, N., Ytlmaz, H., Helps, C.R., Harbour, D.A. (2005). Antibodies to Borna disease virus in subacute sclerosing panencephalitis. Pediatric Infectious Disease Journal, 24(9). 833-4.
- ↑ Thomas, D.Rh., Chalmers, R.M., Crook, B., Stagg, S., Thomas, H.V., Lewis, G., Salmon, R.L., Caul, E.O., Morgan, K.L., Coleman, T.J., Morgan-Capner, P., Sillist, M., Kench, S.M., Meadows, D., and Softley, P. (2005). Borna disease virus and mental health: a cross-sectional study. Quarterly Journal of Medicine, 98. 247-254.
- ↑ Suberbielle, E., Stella, A., Pont, F., Monnet, C., Mouton, E., Lamouroux, L., Monsarrat, B., Gonzalez-Dunia, D. (2008). Proteomic analysis reveals selective impediment of neuronal remodeling upon Borna disease virus infection. Journal of Virology, 82(24). 12265-12279.
- ↑ Lee, B., Matsunaga, H., Ikuta, K., and Tomonaga, K. (2008). Ribavirin inhibits Borna disease virus proliferation and fatal neurological diseases in neonatally infected gerbils. Antiviral Research, 80. 380-384.
 KSF
KSF