Chemical name
 From Citizendium - Reading time: 2 min
From Citizendium - Reading time: 2 min
A chemical name is some formalized description of a molecule, or of certain preparations of a molecule. Such names are to be distinguished from common name, with "sodium chloride solution", or "0.85% sodium chloride in water", being formal while "salt water" is not.
There are additional terms for pharmacologic designations. Nevertheless, the basic chemical terms include:
Molecular description[edit]
Empirical formula[edit]
A notation that identifies the total number of atoms of different elements in a given molecule. Water, for example, has two hydrogen and one oxygen atom, and has the notation H2O. Ethanol is C2H6O, although a slightly modified notation may be used to express substructure: C2H5OH is more common for ethanol, to show that one hydrogen and one oxygen combine in a hydroxyl group.
Formal name[edit]
Using rules defined by the International Union of Pure and Applied Chemistry, the name may be written formally, to provide, within the limits of text, not only the empirical information but information on structure. For example, there are two different molecules that can be formed from three carbons, one oxygen, and eight hydrogens:
- Structural formula CH3CH2CH2OH, also called n-propanol. The name is formal: "propan" is a root indicating a straight chain of three carbon atoms, while "-ol" indicates a hydroxyl group; the combination of a root describing an arrangement of carbon atoms with at least one hydroxyl is an alcohol.
- structural formula CH3CHOHCH2OH, also called iso-propanol.
In these two examples, the prefixes 'iso' and 'N' indicate where the hydroxyl is attached. Even more formal notation disambiguates the atom to which a subgroup is attached, so isopropanol also can be written, but IUPAC rules, as propane-2-ol.
Molecular drawing[edit]
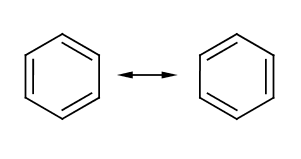
Benzene ring
Especially complex molecules, or where the angle or other two- or three-dimensional relationships are important, may have a structured drawing as an additional descriptor; see benzene.
 KSF
KSF