Explosives
 From Citizendium - Reading time: 12 min
From Citizendium - Reading time: 12 min
| This article may be deleted soon. | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
A chemical explosive is a compound or a mixture of compounds susceptible of a rapid chemical reaction causing a quick physical outburst of gases or heat radiation. The first explosives were created by the Chinese in the 11th century. These were mixtures of nitrate salts, sulfur and charcoal, now known as gunpowder#black gunpowder|black powder. Gunpowder which is a low explosive, exhibits #deflagration|deflagration, or rapid burning, rather than detonation, the reaction exhibited by high explosives. While, perhaps counterintuitively, their explosions are started by chemical explosives, nuclear weapons produce far greater force by totally different mechanisms. Especially in the military, discussions often focus on "explosives and demolitions". The field of demolition, which also applies to civilian construction projects, is the process of destroying things. While one tends to think of blowing up a bridge as a tactical maneuver as part of a countermobility effort, old buildings and roads constantly are being demolished to clear land for new construction. Explosives, especially as used in advanced techniques such as a building implosion, can be more efficient than breaking up structures with tools. There are, however, many explosive applications that do not involve demolition. When it is absolutely, positively essential that an escape hatch opens, or two stages of a space launch vehicle separate, explosive-driven pyrotechnic components are standard engineering components. Explosives are increasingly used as alternatives to large presses in manufacturing, to apply massive directional force or to generate gas, in addition to the traditional fireworks usage of pyrotechnics. This article discusses the characteristics of non-nuclear explosions, which need to be understood for many areas such as emergency management. Actually, a number of characteristics are shared between nuclear and non-nuclear explosions, or between niches, such as high-yield airbursts either nuclear or non-nuclear in nature. Fuzing and initiation[edit]
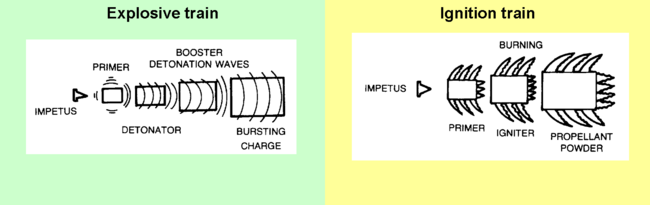
Most devices which contain high explosives, even the relatively low levels of "smokeless" powder used in conventional firearms, involve several stages collectively called the ignition or explosive train. The process is initiated by some event affecting a small amount of a sensitive primary explosive. This event could be a firing pin striking the primer (ammunition)|primer in a rifle cartridge, or a fuze or electrical command setting off a blasting cap or other detonating mechanism in a demolitions charge. Either directly, or through a force-multiplying secondary charge, the detonation wave sets off the main charge. Primary explosives[edit]Initiating the detonation, therefore, usually involves setting off, via a fuze or direct electrical command, a small amount of a sensitive primary explosive such as lead azide (most common), PETN, or DDNP. Black gunpowder is still used as an initiator for artillery propellants. Mercury fulminate is no longer used commercially due to stability problems, but it may be found in improvised explosives since its preparation is simple if risky. Secondary explosives[edit]The primary explosive may have sufficient energy to start the detonation in the main charge of a #tertiary explosive|tertiary explosive such as TNT (explosive)|trinitrotoluene or dynamite. However, with some less sensitive explosives such as ammonium nitrate-fuel oil, an intermediate booster charge made of a selected explosive, such as tetryl, is set off by the detonator producing a stronger detonation wave to trigger the main charge. Tertiary explosives[edit]While primary and secondary explosives are part of the initiation system, tertiary explosives carry out the main purpose of the explosive use. For many years TNT (explosive)|TNT was the most common military explosive, but its use has been decreasing, and it is no longer commercially manufactured in the United States of America. Reasons for the loss of popularity included its effect on the environment, its cost-performance , and the availability of superior insensitive high explosives (IHE) for military uses. In commercial blasting, ammonium nitrate-fuel oil is the most commonly used explosive for commercial applications requiring large quantities. Various dynamites and plastic explosives are used when smaller, more precisely placed charges are needed. Especially after catastrophic fires on aircraft carriers, which led to the detonation of TNT munitions, the U.S. military has been moving to insensitive high explosives. PBXN-109 is most common. TATB has been the IHE in nuclear weapons. Classes by propagation rate[edit]Explosions propagate relatively slowly through gunpowder, which is considered to be a low explosive. The reaction propagates in nanoseconds rather than the milliseconds of low explosive combustion; the approximate boundary between deflagration and detonation is 3300 feet per second. From the detonation, a supersonic shock wave propagates and continues the reaction through the rest of the explosive material. The gases produced are vastly faster than those produced by low explosives. Deflagration of a propellant proceeds in a direction normal to the surface of the gunpowder#grain|propellant grain. Material is consumed in parallel layers, so the geometry of the grain does not change as the burning takes place. Propellant is volatilized by heat transfer from the flame zone which is in the gas phase above the propellant surface. An increase in the ambient pressure causes the flame zone to move closer to the propellant surface. This increases the rate of heat transfer. The more rapid heat transfer causes an increase in the rate of volatilization of the propellant which correspondingly increases the rate of deflagration. If the flow pattern of the hot combustion gases is perturbed and penetrates the flame zone, an increase in the rate of heat transfer may occur. An increase in the flame temperature also causes an increase in the rate of heat transfer. The flame temperature is a function of the propellant composition. Propellants tend to burn more smoothly at high pressure than at low pressure.[1] Low explosives[edit]When gunpowder ignites, the mixture of solids is converted to a large volume of gas moving at high speed. The gases put great pressure on the projectile, accelerating it down the barrel. Longer barrels increase the exit velocity and momentum carried by the bullet or shell. Explosive devices[edit]Especially with military explosives like trinitrotoluene (TNT), the requirement for a detonation wave reduces the chances of accidental denotation. For example, a rifle bullet fired into TNT will not detonate it, unless the TNT is also burning. Insensitive high explosives, however, are much less sensitive to shock than TNT. Hazards[edit]Explosives present various levels of risk probabilites regarding accidental detonation, the intensity of the detonation, and the overall effects of the explosion. A representative classification comes from the U.S. Department of Transportation, used for shipping risk assessment:[2]
Fast propagation[edit]Certain high explosives, such as PETN|pentaerythritol tetranitrate (PETN), have exceptionally high detonation velocity, effectively "instantaneous" at chemical explosive speeds. PETN, in a flexible tube, is generically called detonating cord, or by its early trademark of PrimacordTM. Connections of detonating cord can effectively synchronize several separate explosive charges. Chemistry[edit]Beginning in the 19th century, chemists created many different kinds of explosives for different tasks: warfare, demolition, mining and pyrotechnics. Nitrostarch, first prepared in 1833 by the France|French chemist, Henri Braconnot, is generally considered to be the first high explosive. Nitroglycerin was discovered in 1846 or 1847 by the Italy|Italian, Asconio Sobrero, but his formulation was too unstable to be used until Alfred Nobel found ways to desensitize it.[3] Sweden|Swedish chemist Alfred Nobel (1833-1896) realized that nitroglycerin was too unstable for practical use. But once dissolved in clay and shaped into rods, it made a safe and highly effective explosive, dynamite, that was used primarily in civil engineering. Trinitrotoluene (TNT) was preferable to nitroglycerin-based explosives for field and military use, since it was far more stable and resistant to unintentional detonation. Indeed, there is a class of modern insensitive high explosives that will not explode without deliberate and controlled conditions, even in the violence of an airplane crash. Germany|German chemist Fritz Haber (1868 – 1934) arguably had a greater impact than Nobel. Haber and Carl Bosch discovered a method for nitrogen fixation (converting atmospheric nitrogen to ammonia), thus making inexpensive nitrate salts available for fertilizers and high explosives. This discovery made possible modern agriculture and modern warfare based on high explosives packed into artillery shells. Since Haber also oversaw the German use of poison gas during the World War I, he pioneered the era of weapons of mass destruction. When Haber was awarded the Nobel Prize for Chemistry in 1918 for his work on nitrogen, it was over the objections of some scientists because of his wartime activities. Oxygen balance[edit]One of the measures of explosive effectiveness is its oxygen balance. The oxygen balance of an explosive material is closely related to the explosive power. The oxygen balance is the ratio of oxygen contained in the explosive material to the amount of oxygen required for complete oxidation of the explosive material. Explosive compositions with better oxygen balances are more powerful, but such compositions can be improved by mixing an explosive with a negative oxygen balance with a different substance with a positive balance, or using atmospheric oxygen in a #volumetric explosive|volumetric explosive. New energetic substances[edit]Most current explosives are based on nitro groups, in the molecular classes of aliphatic nitrate esters, nitroaromatics, and nitramines. There are a few non-nitrogen based explosives, such as perchlorates and peroxides. New CHNO/F classes include:[4]
All-nitrogen molecules are being explored. Manufacturing[edit]While many reactions in manufacturing can result in explosions, the products are not intended to explode, putting explosives manufacturing in a special hazard category. Manufacturing plants are often built with a combination of weak and extremely strong walls, to channel blasts in the safest possible direction. Chemical engineering of explosives[edit]Quite a number of reactions, especially nitration, which are routine parts of explosives manufacturing, are Exothermic reaction|exothermic — and the intermediates or products may be heat-sensitive. While small laboratory quantities of nitroglycerine might be made without disaster, this is inconceivable for large-scale production. Glycerine is added to mixed nitric and sulfuric acid, in agitators through which cooling coils, carrying brine chilled to 5 degrees Celsius (unit), hold the reaction below 10 degrees. "Above this temperature there is great danger that the charge will react very violently, ultimately resulting in an explosion."[5] Safety engineering[edit]Especially for military applications, there has been a trend to make insensitive high explosives, which are far less likely to detonate due to fire or impact. The discipline of Hazard from Electromagnetic Radiation to Ordnance deals both with the prevention of explosive initiation purely from radiation affecting the explosive chemical, as well as to other components of a complete weapon. Consequences management[edit]When explosives, or explosive weapons, are variously in accidents, or incompletely detonated, decontamination of scattered material may be necessary, or explosive ordnance disposal may be necessary to render-safe the material. Firefighting involving explosives is difficult enough when both the exact explosive composition and quantities are known as well as established techniques to extinguish a fire involving said explosive. Incorrect information can make matters far worse. For example, the 1947 Texas City explosion began with a fire among ammonium nitrate containers in the hold of a ship. The responders sealed the hold and pumped in steam, which, in many situations, is an effective method for putting out shipboard fires. Unfortunately, with the particular chemicals on board, it provided the conditions to convert a fire into a massive explosion, devastating the city. Fluid dynamics[edit]
Techniques such as flash radiography are giving insight about what actually happens inside an explosion. Transfer of force[edit]Traditionally, the explosive wave was composed of propagation within the solid or liquid explosive, and then by gases moving at high speed. Brisance represents the instantaneous shattering power of the explosive, highly correlated with detonation velocity, while explosive power is the total work done by the explosion's energy integrated over time. Once in air, the force of the blast produced by an explosive is characterized as overpressure; overpressure can be affected by Mach effect reflections of waves. Explosively formed projectiles (EFP), the explosive energy could be carried, and concentrated, by molten metal or a superfast metal mass. Smaller than EFPs, dense inert metal explosives carry the energy in a combination of gas and finely divided, dense metal powder, which shortens the distance that the blast travels but increases the overpressure in that area. Conventional explosives, while they may produce large volumes of gases, propagate their explosion on a wavefront. The wave can be wide, or focused into a small area as with an explosively formed projectile. It can be modeled as a two-dimensional effect moving through a three-dimensional space. Volumetric explosives[edit]As opposed to explosives that are essentially surface reactions, volumetric explosives cause intense, effectively simultaneous, reactions in a three-dimensional space. Some catastrophic accidental explosions, as in coal mines or grain storage, have been triggered when an aerosol particulate distribution forms in an explosive concentration in air. Some terrorists increase the explosive yield of improvised explosive devices by strapping cylinders of liquified natural gas, hydrogen, or acetylene to an explosive charge, but the increase does not begin to approach a fuel-air explosive that meters fuel such that it is in the optimal aerosol size and in stoichiometry|stoichiometric mixture with atmospheric oxygen. Military volumetric explosives are of two types, fuel-air (FAE) and thermobaric (TBE). One might draw a rough analogy between those types, and the difference between a jet engine and a rocket motor: the FAE and the jet depend on atmospheric oxidizer, where the thermobaric and rocket are atmosphere-independent.[7] Not all thermobaric explosives ignore oxygen in their environment; some ground-penetrating bombs with thermobaric warheads deliberately exhaust the oxygen in the target, to asphyxiate those who are not killed by blast and thermal effects. TBE and FAE both achieve their effects through multi-step detonation processes in which an HE bursting charge disperses an oxygen-deficient energetic fuel. They have different pressure-vs-time versus high explosive and one another. Both share the property of needing a very thin walled case, so more of the weapon weight can go into explosive payload.[8] Fuel-air explosive[edit]In an FAE detonation, an HE bursting charge creates an aerosol cloud of fuel (gases, liquid or pulverized powder) mixed with ambient air. A subsequent ignition then generates a 2700+ °F fireball which consumes an enormous quantity of atmospheric oxygen. Because of the requirement to create a finely mixed volatile mixture, an FAE is best employed in the open where it can have its greatest effect against soft targets. If detonated outside a fortification, the aerosol cloud initially generated by an FAE will penetrate small openings and crevices and upon detonation will create a large pressure impulse—effective against unprotected personnel and non-reinforced buildings.[9] In essence, the primary effect of a FAE is its blast effect." Thermobaric explosion[edit]Thermobaric explosives first appeared in Soviet literature in the 1960s, and were deployed in the 1980s. This is actually the military term; the FSU scientific community calls them low-density explosives or metallized volumetric explosives. The fluid dynamics of these explosions was much less understood, in the early 2000s, than FAEs. In particular, there are at least three phenomena: the initial detonation wave, a continued pressure wave, and coupling of the fireball to the target.
"In a TBE detonation, however, an intermediate anaerobic combustion reaction lasting a few hundred microseconds precedes the aerobic combustion of the fuel. (Common powders used as the fuel for TBE include the highly energetic light metals aluminium, titanium, boron, and magnesium.) In this second step, larger fuel particles burn anaerobically and increase the duration of the initial detonation impulse. In the final process, the expanding shockwave heats and mixes the fuel-rich air, igniting the volatile atmosphere at 3000 meters per second and further extending the pressure impulse duration. The resulting 5400+° F fireball and 29-atmosphere pressure wave lasts longer than a conventional blast-fragmentation warhead, resulting in much higher temperatures and a destructive pressure wave. The primary working mechanisms of TBE weapons is to create high thermal radiation and pressure in an enclosed area followed by a deep vacuum." Enhanced thermal effect[edit]Some explosions also carry significant heat and will also have incendiary effects; combined explosive-incendiary effects may also be a result of the design of the container (e.g., high explosive inside a zirconium, magnesium or other highly combustible casing. Pyrotechnics[edit]Pyrotechnics, originally the art and craft of fireworks, involve explosives which react at low rates. Modern pyrotechnic applications include delay fuses and various explosively driven fasteners -- the explosive may drive a fastener into a desired material (e.g., a nail into concrete) or can disconnect critical fasteners, such as on an escape hatch. The colorful flames of fireworks are created by adding different mixtures of elements which, when heated by the explosives, become incandescent. In general, technological change has been less in fireworks than in other types of pyrotechnics.[11] Modern pyrotechnics have expanded to become engineering tools. A pyrotechnic fastener, for example, contains a very small explosive charge, which shatters it. Pyrotechnic bolts, for example, are used in breaking high-strength mechanical connections under stress, such as the interstage fastenings of the stages of a multistage rocket. References[edit]
|
|||||||||||||||||
 KSF
KSF