Magnetization
 From Citizendium - Reading time: 10 min
From Citizendium - Reading time: 10 min
Magnetization, M, is the magnetic moment per unit volume, V of a material, defined in terms of the magnetic moments of its constituents by:
where the magnetic moment mj of the j-th constituent is a vector property that determines the torque the object experiences in a magnetic field tending to align its moment with the field. Here, N is the number of magnetic moments in the volume V. The M-field is measured in amperes per meter (A/m) in SI units.[1] Usually the magnetization is referred to a particular location r by imagining the volume V to be a microscopic region enclosing point r, and is anticipated to change with time t in the general case (perhaps because the moments are moving), defining a magnetization field, M(r, t).[2]
At a microscopic level, the origin of the magnetic moments responsible for magnetization is traced to angular momentum, such as due to motion of electrons in atoms, or to spin, such as the intrinsic spin of electrons or atomic nuclei. According to the Bohr-van Leeuwen theorem. magnetism is a quantum mechanical effect; on the basis of classical mechanics, there cannot be diamagnetism, paramagnetism or collective magnetism (that is, most notably ferromagnetism or antiferromagnetism, but also ferrimagnetism and metamagnetism).[3] As stated by Van Vleck: "At any finite temperature, and in all finite applied electrical or magnetic fields, the net magnetization of a collection of nonrelativistic classical electrons in thermal equilibrium vanishes identically."[4]
Magnetic moments[edit]
As magnetization is related to magnetic moments, its understanding requires a notion of where magnetic moments originate. As a general statement, magnetic moments of objects composing matter are related to either angular momentum or to spin, both of which at a microscopic level are related to rotational phenomena.[5] The connection is made via the gyromagnetic ratio, the proportionality factor between magnetic moment and spin or angular momentum for a given object.
These ideas apply to nucleii and other particles, but a discussion focused on electrons in atoms can be found in the article magnetic moment. The magnetic moment mS of a system of electrons with spin S is:
and the magnetic moment mL of an electronic orbital momentum L is:
Here the factor mB refers to the Bohr magneton, defined by:
with e = the electron charge, ℏ = Planck's constant divided by 2π, and me = the electron mass. These relations are generalized using the g-factor:
with g=2 for spin (J = S) and g=1 for orbital motion (J = L).[6] The resultant total spin S of an ensemble of electrons in an atom is the vector sum of the constituent spins sj:
Likewise, the orbital momenta of an ensemble of electrons in an atom add as vectors.
Where both spin and orbital motion are present, they combine by vector addition:
and the magnetic moment is
with g now the Landé g-factor or spectroscopic splitting factor:[7]
If a collection of atoms with these associated magnetic moments are now subject to a magnetic flux, all the atoms will experience a torque, in part due to the applied field and in part as a response to the magnetic flux they create among themselves. The calculation of the magnetization thus involves determination of the orientation of these moments taking into account their influence upon each other and also the influence of the external magnetic flux.
It should be noted that in some solids the electronic structure is very distorted from that of free atoms. In such materials magnetism is discussed using the electronic band structure of the solid, and one refers to "band magnetism" or "itinerant electron magnetism".[8]
Types of magnetization[edit]
The major magnetic forms are paramagnetism, ferromagnetism and diamagnetism.
Paramagnetism[edit]
When an assembly of atoms is placed in a magnetic flux, a torque is exerted upon the atoms because of their magnetic moment. The result is that atoms that are aligned with the magnetic flux have lower energy than those that are at an angle to the field. The difference in energies is proportional to the magnetic flux density and to the component of magnetic moment along the field.
According to the Boltzmann factor, higher energy configurations are less probable than lower ones, and as temperature is lowered, the population of the lower energy configurations grows at the expense of the higher energy configurations. Also, as the magnetic flux density is increased the separation of the configurations increases and the lower energies become more populated.[9] These observations are made quantitative in the Brillouin theory of paramagnetism. This theory employs the energy of a magnetic moment m in an applied magnetic field H:
with m an integer between m = −J and m = J and g the Landé spectroscopic splitting factor. We use Boltzmann statistics to state the probability that a magnetic moment has this energy is proportional to exp(-E/kBT), where kB is the Boltzmann constant and T is the absolute temperature. Summing over the allowed values of magnetic moment, the magnetization in the direction of the magnetic field is found to be:[10]
with N the volume density of atoms, J the angular momentum quantum number, and
known as the Brillouin function, with argument x given by:
In most cases, H is the externally applied field, but in some cases an internal field contribution is present, complicating the energy spectrum.
Interacting atoms[edit]
The outline of paramagnetism above ignores all magnetic interactions between atoms, and makes them all act individually.[11] A natural question is: if the torque aligning atoms is due to the magnetic field in the atom's vicinity, shouldn't the field include the effect of the neighboring atoms upon the field?
Such a modified theory was proposed by Pierre-Ernest Weiss by introducing the notion of a molecular field, a magnetic field contribution that was proportional to the magnetization in the vicinity of an atom:[12]
where HW is the "Weiss field" and γ is the "molecular field constant". This contribution is added to the applied magnetic field HA to obtain the total field H:
Given a method to determine M from H when there is no Weiss field (no interactions), that is, a function M = M(H), we then find (approximately) with the interactions present:
an implicit determination of M for any given HA. In particular, if we adopt the approach based upon the Brillouin function BJ as a function of
all that is needed is to replace H with the modified H above that includes the Weiss field.
This approach has some application to those paramagnetic and ferrimagnetic materials that are ionic solids with localized moments.[13] It doesn't work for ferromagnetic materials because most are metals with itinerant (not localized) electrons, and because these materials exhibit spontaneous magnetization without any need for an applied field.
Ferromagnetism[edit]
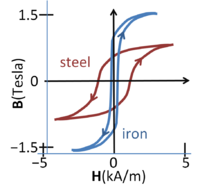
Magnetic flux density vs. magnetic field in steel and iron; the curve depends upon the direction of traversal, the phenomenon of hysteresis
For ferromagnetic materials, the self-interaction of the atoms tends to align them even when no external magnetic field is present. As a result, ferromagnetic materials create a net magnetic field (and magnetic flux density) in the space surrounding the material, and can form permanent magnets at temperatures below the Curie temperature of the material. At higher temperatures, the aligning interaction is inadequate to overcome the randomness introduced by thermal motions, and the material becomes paramagnetic.
The basis for cooperation between atomic magnetic moments is that electrons obey the Pauli exclusion principle that no two can occupy the same quantum state. That means configurations with aligned spins are energetically favored over misaligned spins by an exchange interaction, favoring magnetization. Technically speaking, the Pauli principle requires a spatial part of the wavefunction that is antisymmetric for parallel spins, which keeps the mutually repulsive electrons apart, lowering their interaction energy. In some cases, the balance with the change in kinetic energy favors the antiparallel case (antiferromagnetism), but in ferromagnetism the parallel configuration is favored.[14] The same idea underlies Hund's rules for atoms, namely, other things equal, electrons in atoms populate states to maximize their total spin. Not addressed here is the unanswered question: why do ferromagnetic materials profit from this effect more than other materials?[15]
The figure shows magnetization curves for two different ferromagnetic materials. Notice that H=0 does not mean B=0, or vice versa. The curves exhibit hysteresis, that is, the curve is history dependent and, in particular, depends upon the direction in which the magnetic field increases. This complex behavior indicates that magnetization in such materials is not an equilibrium process. Larger samples break up into magnetic domains or sub-regions of different magnetization directions separated by domain walls.[16] The magnetization curve is affected (in part) by the change in size of the various domains as they reluctantly adapt to changes in the external field.[17] At high H-fields, the curves saturate as the domains aligned with the field dominate, and may coalesce into a single monodomain.
Today it is still impossible to predict from first principles that iron is ferromagnetic.[18] However, some guidance can be obtained as to which metals are candidates, and which are not, based upon estimates of how exchange energy varies with atomic radii and spacing. "The theory of magnetism in solids is one of the central challenges in condensed matter physics, intrinsically involving many-body correlations, long range order and phase transitions..."[19]
Diamagnetism[edit]
Diamagnetism is the reduction within a material of an external magnetic flux by the reaction of the material. The perfect diamagnetic material is the superconductor, which completely suppresses the magnetic field inside itself in what is called the Meissner effect.[20] Unlike the other examples of magnetic behavior considered here, superconductivity is the consequence of macroscopic currents, not atomic level magnetic moments.
All materials exhibit diamagnetism, but it ordinarily is so weak that it is apparent only in materials where no other form of magnetic behavior is evident, like the noble gases or some diatomic gases .[21]
Magnetization and magnetic field[edit]
In principle, the magnetic flux density B can be found by observing the force F on a test charge of size q moving with velocity v using the formula:
With B in hand, the magnetization could then be determined from Maxwell's equations using:
where j is the sum of conduction and polarization current densities. In practice, things are done differently. The magnetic field or magnetic intensity H is introduced with the definition:[22]
It is useful to note that, unlike B which has zero divergence,
which is not zero unless it happens that divM = 0.[23] In addition, B and H satisfy different conditions on boundaries between media.
The magnetization M is calculated in terms of H using a so-called constitutive relation calculated by the techniques of condensed matter physics. The simplest example calculates the magnetic susceptibility χm defined as:
(which is dimensionless) and a magnetic permeability by:
whereupon the magnetic flux density is determined as:
Here, χm < 0 corresponds to diamagnetism, and χm > 0 to paramagnetism. In ferromagnetic materials χm is a function of H even at low values of H; in paramagnetic and diamagnetic materials the dependence of B upon H departs from a linear to a saturated behavior only at high fields.[24]
Notes[edit]
- ↑ Units for Magnetic Properties. Lake Shore Cryotronics, Inc.. Retrieved on 2010-12-09.
- ↑ The notion of a "physically infinitely small volume" is introduced, which means a volume small enough that macroscopic variables are effectively constant within it, but large enough that averaging over the volume eliminates atomic level fluctuations.Dmitri Berkov (2007). “§1.2 Basic physical principles”, Wilfried Andrä, Hannes Nowak, editors: Magnetism in Medicine: A Handbook, 2nd ed. Wiley-VCH, p. 38. ISBN 3527405585.
- ↑ According to Patrik Fazekas (1999). Lecture notes on electron correlation and magnetism. World Scientific, p. 42. ISBN 9810224745. “...first derived by N. Bohr in a thesis work (Copenhagen 1911). J.H. Van Leeuwen rediscovered and thoroughly discussed it in her thesis work (Leiden, 1919). The theorem became widely known only when Van Vleck re-examined it in his book The Theory of Electric and Magnetic Susceptibilities, first published in 1932”
- ↑ This statement by Van Vleck takes on a variety of forms. A more extended discussion is found as a footnote in Paweł Haensel, A. Y. Potekhin, D. G. Yakovlev (2006). “Equilibrium plasma properties: outer envelopes”, Neutron stars: Equation of state and structure. Springer, pp. 54-55. ISBN 0387335439.
- ↑ Besides the microscopic constituents of matter, other objects, like a wire loop carrying a current, can have a magnetic moment, and will experience a torque in a magnetic field (as in an electric motor). That is not the topic here.
- ↑ Charles P. Poole (1996). Electron spin resonance: a comprehensive treatise on experimental techniques, Reprint of Wiley 1982 2nd ed. Courier Dover Publications, p. 4. ISBN 0486694445.
- ↑ R. B. Singh (2008). Introduction To Modern Physics. New Age International, p. 262. ISBN 8122414087.
- ↑ J Kübler (2009). “§1.3 How to proceed”, Theory of itinerant electron magnetism, Revised ed. Oxford, pp. 25 ff. ISBN 0199559023.
- ↑ Charles Kittel (2004). “Quantum theory of paramagnetism”, Introduction to Solid State Physics, 8th ed. Wiley, pp. 303 ff. ISBN 978-0-471-41526-8.
- ↑ K. H. J. Buschow, Frank R. Boer (2003). “Chapter 3: Paramagnetism of free ions”, Physics of magnetism and magnetic materials. Springer, p. 13. ISBN 0306474212.
- ↑ The atoms are expected to exchange energy, however, so thermal equilibrium can be established.
- ↑ Nicola A Spaldin (2010). “§5.2 The Curie-Weiss law”, Magnetic materials: fundamentals and applications, 2nd ed. Cambridge University Press, p. 53. ISBN 0521886694.
- ↑ Chapter 9 of reference cited above: Nicola A Spaldin (2010). “Chapter 9: Ferrimagnetism”, Magnetic materials: fundamentals and applications, 2nd ed. Cambridge University Press, pp. 113 ff. ISBN 0521886694.
- ↑ Martina Müller (2007). “§2.1.1 Electronic structure of 3d ferromagnets”, Electronic Structure of Ferromagnet-Insulator Interfaces: Fe/MgO and Co/MgO. Forschungszentrum Jülich, p. 10. ISBN 3893364935.
- ↑ For more about the exchange interaction, see LD Landau and EM Lifshitz (1960). “Chapter V: Ferromagnetism”, Electrodynamics of continuous media. Pergamon Press, pp. 146 ff. , and for some simple examples Assa Auerbach (1999). “Chapter 2: Spin exchange”, Interacting electrons and quantum magnetism. Springer, pp. 11 ff. ISBN 0387942866. .
- ↑ F Fiorillo, C Appino and M Pasquale (2005). “§1.3 Energy in a magnetic system. Domain walls and domain structures”, Isaak D. Mayergoyz, Giorgio Bertotti, editors: The Science of Hysteresis, volume III. Elsevier Academic Press, pp. 29 ff. ISBN 0123694337.
- ↑ Same reference as previously, but p. 169:J Kübler (2009). “§4.1.1 Stoner theory”, Theory of itinerant electron magnetism, Revised ed. Oxford, pp. 169 ff. ISBN 0199559023.
- ↑ Bernard Dennis Cullity, Chad D. Graham (2009). “Chapter 4: Ferromagnetism”, Introduction to magnetic materials, 2nd ed. Wiley-IEEE, p. 131. ISBN 0471477419.
- ↑ Richard M Martin (2004). Electronic structure: basic theory and practical methods. Cambridge University Press, p. 24. ISBN 0521782856.
- ↑ Toshihiko Tsunetō (1998). “§1.1.3 Perfect diamagnetism”, Superconductivity and superfluidity. Cambridge University Press, pp. 4 ff. ISBN 0521570735.
- ↑ Nicola A. Spaldin (2010). “§4.3 Diamagnetic substances”, Magnetic Materials: Fundamentals and Applications, 2nd ed. Cambridge University Press, pp. 41 ff. ISBN 0521886694.
- ↑ To deal with a quantity with the same dimensions as the magnetic flux density, some authors introduce the magnetic polarization, symbol J, as J = μ0 M so B = J + μ0H. See for example, K. H. J. Buschow, Frank R. Boer (2003). Physics of magnetism and magnetic materials. Springer, p. 80. ISBN 0306474212. Alex Hubert, Rudolf Schäfer (1998). Magnetic domains: the analysis of magnetic microstructures. Springer, p. 11. ISBN 3540641084.
- ↑ DJ Griffiths (1999). “§6.3.2 A deceptive parallel”, Introduction to electrodynamics, 3rd ed. Prentice Hall, p. 273. ISBN 0138053260.
- ↑ David Jiles (1998). “§4.4.3 Field dependence of paramagnetic susceptibility”, Introduction to magnetism and magnetic materials, 2nd ed. CRC Press, p. 105. ISBN 0412798603.
 KSF
KSF