Meta-analysis
 From Citizendium - Reading time: 19 min
From Citizendium - Reading time: 19 min
Meta-analysis is defined as "a quantitative method of combining the results of independent studies (usually drawn from the published literature) and synthesizing summaries and conclusions which may be used to evaluate therapeutic effectiveness, plan new studies, etc., with application chiefly in the areas of research and medicine."[1]
A meta-analyses is a subset of systematic reviews in which the results of the studies are numerically pooled.
Standards for the reporting of meta-analyses exist.[2]
Validity of meta-analysis[edit]
Studies on the validity of meta-analyses conflict.[3][4][5] Some of the conflict may be due to the methods used to compare the meta-analyses.[6]
Methods of meta-analysis[edit]
Guidelines are available for the conduct[7] and reporting[2] of meta-analyses.
Searching for studies[edit]
Meta-analyses vary in the extent of their searches for underlying studies. [8] No individual database contains all the existing randomized controlled trials; however, the Cochrane database may be the most comprehensive.[8]
Machine learning and text categorization has been use for searching.[9][10]
There is not a consensus on what details of searching should be reported in a meta-analysis.[11]
Stopping rules[edit]
There is debate on how extensive should be the search for studies as there is are diminishing returns with extensive searching. Some studies suggest limiting searches[12][13][14] while other studies advocate exhaustive searches[15][16][17][18][19][20] including unpublished studies[21][22]. The role of databases other than MEDLINE is not clear.[23]
Various methods have been proposed for when to stop searchers.[24][25][26]
Selecting studies for inclusion[edit]
Conflict in selection of trials to be included in the meta-analysis can affect the conclusions of a meta-analysis.[27][28][29]
Although meta-analyses in general are very inclusive, arguments exist for only including the best trials.[30]
Assessing the quality of trials[edit]
Assessing the quality of a trial by only using the published report may lead to inaccurate conclusions.[31]
The formal methods below have some difficulty with reproducibility.[32]
Cochrane bias scale[edit]
The Cochrane Collaboration uses a six item tool.[33]
Jadad score[edit]
The Jadad score may be used to assess quality and contains three items:[34]
- Was the study described as randomized (this includes the use of words such as randomly, random, and randomization)?
- Was the study described as double blind?
- Was there a description of withdrawals and dropouts?
Each question is scored one point for a yes answer. In addition, for questions and 2, a point is added if the method was appropriate and a point is deducted if the method is not appropriate (e.g. not effectively randomized or not effectively double-blinded).
Statistical methods[edit]
Measuring consistency of study results[edit]
Consistency can be statistically tested using either the Cochran's Q or I2.[35][36] The I2 is the "percentage of total variation across studies that is due to heterogeneity rather than chance."[35] These numbers are usually displayed for each group of studies on a Forest plot.
In interpreting of the Cochran's Q, heterogeneity exists if its p-value is < 0.05 or possibly if < 0.10[37][38].
The following has been proposed for interpreting I2:[35]
- Low heterogeneity is I2 = 25%
- Moderate heterogeneity is I2 = 50%
- High heterogeneity is I2 = 75%
or according to the Handbook of the Cochrane Collaboration:[39]
- 0%-40%: might not be important
- 30%-60%: may represent moderate heterogeneity
- 50%-90%: may represent substantial heterogeneity
- 75%-100%: considerable heterogeneity
However, I2, even when the value is 0%, can be misleading if the confidence intervals around the value are not provided.[40][41]
Statistical methods exist for assessing the importance of subgroups.[42]
Comparing rates of dichotomous outcomes[edit]
Studies are usually statistically combined by a method such as the DerSimonian and Laird.[43] The DerSimonian and Laird weight for pooling studies is a type of inverse variance weight and creates a random effect model. Determining prediction intervals for random effects may help apply results to clinical practice.[44]
Statistical packages are available from the Cochrane Collaboration (http://www.cc-ims.net/revman) and for R (programming language) (rmeta and HSAUR2).
Studies with groups having zero events[edit]
Excluding studies with zero events total events (zero-total-event trials) or zero events in one treatment group (zero-event trials) may exaggerate effect sizes.[45][46] An alternative is to use a continuity correction.[47] Rather than using a constant continuity correction, less bias may occur by correcting with either[48]
- "empirical estimate of the pooled effect size from the remaining studies in the meta-analysis."
- "a function of the reciprocal of the opposite group arm size"
For an example of continuity correction using the second method above:[45]
- S is the sum of corrections for event and no event cells (usually S=1 in a zero-event trial and S=2 in a zero-total-event trial)
- R is the ratio of group sizes (R=1 if both groups are the same)
- For a zero-event trial with equal group sizes
- The correction in the larger experimental group is R/S*(R + 1). This becomes 1/1*(1 + 1) = 1
- The correction in the smaller experimental group is 1/S*(R + 1). This becomes 1/1*(1 + 1) = 1
- For a zero-event-total trial with equal group sizes
- The correction in the larger experimental group is R/S*(R + 1). This becomes 1/2*(1 + 1) = 0.5
- The correction in the smaller experimental group is 1/S*(R + 1). This becomes 1/2*(1 + 1) = 0.5
Comparing rates of continuous outcomes[edit]
The standardized mean difference (SMD), also called the effect size (ES), is used. The SMD is:[49]
In the interpretation of SMD:[50]
- 0.2 represents a small effect
- 0.5 a moderate effect
- 0.8 a large effect
Transforming standardized mean difference to odds ratio[edit]
Subgroup analysis[edit]
There are two types of interactions:[53]
- Qualitative interaction interaction exists if the direction of effect is reversed in subgroups.
- Quantitative interaction is when the size of the effect varies but not the direction.
If the subgrouping accounts for all heterogeneity, interaction can be sought using an inverse-variance method for a fixed-effect model.[54]
If the subgrouping does not account for all heterogeneity, interaction can be tested with meta-regression to avoid false-positive results.[54][55] Metagression is detailed in a section below.
Software[edit]
Software available for meta-analysis includes:[56]
- Review Manager (RevMan) by the Cochrane Collaboration
- Meta-analyst[57]
- R programming language:
- metaLik package with reference manual. Allows for the profile likelihood for computing confidence bounds.[58]
- rmeta package with reference manual
- metafor package with manual [59]
- HSAUR2 interactive package with a chapter containing sample demonstrations, "Meta-Analysis: Nicotine Gum and Smoking Cessation and the Effcacy of BCG Vaccine in the Treatment of Tuberculosis" in "A Handbook of Statistical Analyses Using R".[60]
- For meta-analysis of diagnostic tests
- bamdit. "Bayesian meta-analysis of diagnostic test data based on a scale mixtures bivariate random-effects model. Summary statistics are based on pooled and predictive sensitivities and specificities."
- HSROC. " implements a model for joint meta-analysis of sensitivity and specificity of the diagnostic test under evaluation, while taking into account the possibly imperfect sensitivity and specificity of the reference test. This hierarchical model accounts for both within and between study variability. Estimation is carried out using a Bayesian approach, implemented via a Gibbs sampler."
Displaying results[edit]
Study results may be grouped and displayed with a Forest plot.
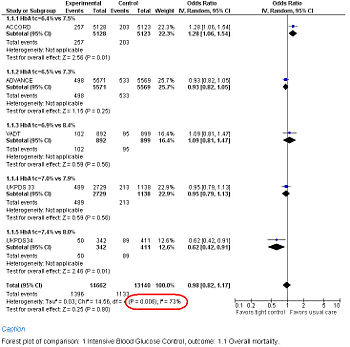
Forest Plot showing meta-analysis of randomized controlled trials of differing target glucose control and mortality for diabetes mellitus type 2. Note the heterogeneity (P<0.05 and high I2 in circled in red) due to increased death when the glycosylated hemoglobin A (Hb A1c) target was 6.0% in the ACCORD trial[61]
Variations on meta-analysis[edit]
Cumulative meta-analysis[edit]
Cumulative meta-analysis has been used to show that 25 off 33 randomized controlled trials of streptokinase not necessary[62] and have shown the delay in adoption of evidence by experts[63].
Cumulative meta-analyses may be prone to false positive results due to repeated tests of statistical significance.[64] This may be avoided by use of trial sequential analysis.[64][65][66]
Individual patient data meta-analysis[edit]
An individual patient data meta-analysis is "where analyses are done using original data and outcomes for each person enrolled in relevant studies; these results are then pooled in one analysis as if patients were in a single large study."[67]
Standards exist for conduct and reporting.[68]
Individual patient data meta-analysis (IPD meta-analysis) may have more long lasting results than other meta-analyses.[69]
Living or continuous meta-analyses[edit]
Because of the above problems the need for living meta-analyses has been made.[70][71] Websites to support collaborative meta-analyses is available.
- Systematic Review Data Repository of the United States of America Agency for Healthcare Research and Quality
- OpenMetaAnalysis at OpenCPU
Meta-regression[edit]
Meta-regression allows simultaneous comparison of multiple sources of heterogeneity.[72][73][74][75]
Meta-regression can examine relationships between predictor and outcome variables including non-linear relationships.[76]
Meta-regression can analyze subgroups.[54]A permutation test may reduce the chance of a false positive subgroup analysis.[55]
When analyzing a meta-regression of dichotomous independent variables, the "results of meta-regression analyses are most usefully expressed as ratios of odds ratios (or risk ratios)."[7]
Meta-regression can be performed with the rmeta package[77] of the R programming language as described by Everitt and Hothorn[78][79].
Meta-regression is not as powerful as individual patient data meta-analysis[80], especially when the distributions of covariates are heterogeneous across studies[81].
Examples of meta-regression analysis are:
- McAlister FA, Wiebe N, Ezekowitz JA, Leung AA, Armstrong PW (2009). "Meta-analysis: beta-blocker dose, heart rate reduction, and death in patients with heart failure.". Ann Intern Med 150 (11): 784-94. PMID 19487713.
- Briel M, Ferreira-Gonzalez I, You JJ, Karanicolas PJ, Akl EA, Wu P et al. (2009). "Association between change in high density lipoprotein cholesterol and cardiovascular disease morbidity and mortality: systematic review and meta-regression analysis.". BMJ 338: b92. DOI:10.1136/bmj.b92. PMID 19221140. PMC PMC2645847. Research Blogging.
- Emerging Risk Factors Collaboration. Erqou S, Kaptoge S, Perry PL, Di Angelantonio E, Thompson A et al. (2009). "Lipoprotein(a) concentration and the risk of coronary heart disease, stroke, and nonvascular mortality.". JAMA 302 (4): 412-23. DOI:10.1001/jama.2009.1063. PMID 19622820. Research Blogging.
Network meta-analysis[edit]
A network meta-analysis[82] and Bayesian hierarchical models[83] pool studies in order to compare to treatments that have not been directly compared.[84][85] Network meta-analyses are commonly not well performed.[86] Network meta-analyses of both randomized controlled trials[87][88][89] and diagnostic test assessments[90] can have misleading results. Network meta-analyses have been conducted by the Cochrane Collaboration.[91][92]
Network meta-analyses can be conducted with Bugs and OpenBugs software.
Meta-analysis of diagnostic tests[edit]
Standards exists for the meta-analysis of diagnostic tests.[93][94] The traditional summary receiver operating characteristic curve (SROC curve) should be replaced by either the hierarchical summary receiver operating characteristic curve(HSROC curve).[94][95] or bivariate random-effects model.[96] Discussions of HSROC and bivariate random-effects meta-analysis are available.[97][96] An example of a meta-analysis using bivariate mixed-effects binomial regression model is available.[98] Examples of using the HSROC and diagnostic odds ratio are available.[99]
Update of existing a systematic review[edit]
Reviewers may elect to conserve resources by updating an existing review.[100]
Overview of reviews[edit]
A systematic review may review other systematic reviews. The reviews may address different treatments of the same disease or different diseases that can be treated with an intervention.[101]
Assessing the quality of meta-analysis[edit]
The quality of a meta-analysis can be assess with:[100]
Factors associated with higher quality meta-analyses[edit]
Meta-analyses by the Cochrane Collaboration tend to be of higher quality.[104]
Individual data meta-analyses, in which the records from individual patients are pooled together into one dataset, tend to have more stable conclusions.[69]
Factors associated with lower quality meta-analyses[edit]
About a third of meta-analyses that happen to precede large randomized controlled trials will conflict with the results of the trial.[3]
Conflict of interest[edit]
Meta-analyses produced with a conflict of interest are more likely to interpret results as positive.[105]
Small study effect and publication bias[edit]
The small study effect is the observation that small studies tend to report more positive results.[106][107][108] This is especially a threat when the original studies in a meta-analysis are less than 50 patients in size.[109]
Publication bias against negative studies is part of the small study effect and may threaten the validity of meta-analyses that are positive and all the studies included within the meta-analysis are small.[110][111]
In performing a meta-analysis, a file drawer[112]or a funnel plot analysis[113][111][114] may help detect underlying publication bias among the studies in the meta-analysis.
Outcome reporting bias[edit]
Meta-analyses in which a smaller proportion of included trials provide raw data for inclusion in the meta-analysis are more likely to be positive.[115] This may be due a bias against reporting negative results.[116]
Problems with meta-analyses[edit]
Disagreement with major clinical trials[edit]
Meta-analyses may not agree with major clinical trials.[3][5][4][117] Some of the disagreement may be due to the methods used in selecting and comparing meta-analyses and trials.[6] Publication bias may be a factor.[118]
The disagreements lead to debate as to whether truth is the meta-analysis or a dominant, large trial.[119]
Conflict of interest[edit]
Meta-analyses may not consider or report conflict of interests among the studies included in the analysis.[120]
Obsolescent meta-analyses[edit]
The conclusions of meta-analyses may be mitigated by research published after the search date of the meta-analysis. This may occur by the time the meta-analysis has been published.[121][122] Strategies have been developed for identifying potentially outdated analyses[123] and their updating[124].
Small meta-analyses more be prone to obsolescence and disagreement with larger, subsequent trials.[125][111]
Publication bias[edit]
Prospero is prospective registry of systematic reviews to reduce publication bias.
Redundant meta-analyses[edit]
Overlapping and redundant meta-analyses have become a problem.[126]
References[edit]
- ↑ National Library of Medicine. Meta-analysis. Retrieved on 2007-12-06.
- ↑ 2.0 2.1 Moher D, Liberati A, Tetzlaff J, Altman DG (July 2009). "Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement". Ann. Intern. Med.. PMID 19622511. [e]
- ↑ 3.0 3.1 3.2 LeLorier J, Grégoire G, Benhaddad A, Lapierre J, Derderian F (August 1997). "Discrepancies between meta-analyses and subsequent large randomized, controlled trials". N. Engl. J. Med. 337 (8): 536–42. DOI:10.1056/NEJM199708213370806. PMID 9262498. Research Blogging.
- ↑ 4.0 4.1 Villar J, Carroli G, Belizán JM (March 1995). "Predictive ability of meta-analyses of randomised controlled trials". Lancet 345 (8952): 772–6. PMID 7891492. [e]
- ↑ 5.0 5.1 Cappelleri JC, Ioannidis JP, Schmid CH, et al (1996). "Large trials vs meta-analysis of smaller trials: how do their results compare?". JAMA 276 (16): 1332–8. DOI:10.1001/jama.1996.03540160054033. PMID 8861993. Research Blogging.
- ↑ 6.0 6.1 Ioannidis JP, Cappelleri JC, Lau J (April 1998). "Issues in comparisons between meta-analyses and large trials". JAMA 279 (14): 1089–93. DOI:10.1001/jama.279.14.1089. PMID 9546568. Research Blogging.
- ↑ 7.0 7.1 The Cochrane Collaboration. Cochrane Handbook
- ↑ 8.0 8.1 Royle P, Milne R (2003). "Literature searching for randomized controlled trials used in Cochrane reviews: rapid versus exhaustive searches.". Int J Technol Assess Health Care 19 (4): 591-603. PMID 15095765.
- ↑ Wallace BC, Trikalinos TA, Lau J, Brodley C, Schmid CH (2010). "Semi-automated screening of biomedical citations for systematic reviews.". BMC Bioinformatics 11: 55. DOI:10.1186/1471-2105-11-55. PMID 20102628. PMC PMC2824679. Research Blogging.
- ↑ Cohen AM, Hersh WR, Peterson K, Yen PY (2006). "Reducing workload in systematic review preparation using automated citation classification.". J Am Med Inform Assoc 13 (2): 206-19. DOI:10.1197/jamia.M1929. PMID 16357352. PMC PMC1447545. Research Blogging.
- ↑ Sampson M, McGowan J, Tetzlaff J, Cogo E, Moher D (2008). "No consensus exists on search reporting methods for systematic reviews.". J Clin Epidemiol 61 (8): 748-54. DOI:10.1016/j.jclinepi.2007.10.009. PMID 18586178. Research Blogging.
- ↑ Stevinson C, Lawlor DA (2004). "Searching multiple databases for systematic reviews: added value or diminishing returns?". Complement Ther Med 12 (4): 228-32. DOI:10.1016/j.ctim.2004.09.003. PMID 15649836. Research Blogging.
- ↑ Fergusson D, Laupacis A, Salmi LR, McAlister FA, Huet C (2000). "What should be included in meta-analyses? An exploration of methodological issues using the ISPOT meta-analyses.". Int J Technol Assess Health Care 16 (4): 1109-19. PMID 11155831.
- ↑ Hopewell S, Clarke M, Lefebvre C, Scherer R (2007). "Handsearching versus electronic searching to identify reports of randomized trials.". Cochrane Database Syst Rev (2): MR000001. DOI:10.1002/14651858.MR000001.pub2. PMID 17443625. Research Blogging.
- ↑ Lemeshow AR, Blum RE, Berlin JA, Stoto MA, Colditz GA (2005). "Searching one or two databases was insufficient for meta-analysis of observational studies.". J Clin Epidemiol 58 (9): 867-73. DOI:10.1016/j.jclinepi.2005.03.004. PMID 16085190. Research Blogging.
- ↑ Avenell A, Handoll HH, Grant AM (2001). "Lessons for search strategies from a systematic review, in The Cochrane Library, of nutritional supplementation trials in patients after hip fracture.". Am J Clin Nutr 73 (3): 505-10. PMID 11237924.
- ↑ Crumley ET, Wiebe N, Cramer K, Klassen TP, Hartling L (2005). "Which resources should be used to identify RCT/CCTs for systematic reviews: a systematic review.". BMC Med Res Methodol 5: 24. DOI:10.1186/1471-2288-5-24. PMID 16092960. PMC PMC1232852. Research Blogging.
- ↑ Wilkins T, Gillies RA, Davies K (2005). "EMBASE versus MEDLINE for family medicine searches: can MEDLINE searches find the forest or a tree?". Can Fam Physician 51: 848-9. PMID 16926954. PMC PMC1479531.
- ↑ Savoie I, Helmer D, Green CJ, Kazanjian A (2003). "Beyond Medline: reducing bias through extended systematic review search.". Int J Technol Assess Health Care 19 (1): 168-78. PMID 12701949.
- ↑ Whiting P, Westwood M, Burke M, Sterne J, Glanville J (2008). "Systematic reviews of test accuracy should search a range of databases to identify primary studies.". J Clin Epidemiol 61 (4): 357-364. DOI:10.1016/j.jclinepi.2007.05.013. PMID 18313560. Research Blogging.
- ↑ Hopewell S, McDonald S, Clarke M, Egger M (2007). "Grey literature in meta-analyses of randomized trials of health care interventions.". Cochrane Database Syst Rev (2): MR000010. DOI:10.1002/14651858.MR000010.pub3. PMID 17443631. Research Blogging.
- ↑ McAuley L, Pham B, Tugwell P, Moher D (2000). "Does the inclusion of grey literature influence estimates of intervention effectiveness reported in meta-analyses?". Lancet 356 (9237): 1228-31. DOI:10.1016/S0140-6736(00)02786-0. PMID 11072941. Research Blogging.
- ↑ Kassaï B, Sonié S, Shah NR, Boissel JP (2006). "Literature search parameters marginally improved the pooled estimate accuracy for ultrasound in detecting deep venous thrombosis.". J Clin Epidemiol 59 (7): 710-4. DOI:10.1016/j.jclinepi.2005.09.013. PMID 16765274. PMC PMC2670362. Research Blogging.
- ↑ Booth A (2010). "How much searching is enough? Comprehensive versus optimal retrieval for technology assessments.". Int J Technol Assess Health Care 26 (4): 431-5. DOI:10.1017/S0266462310000966. PMID 20923586. Research Blogging.
- ↑ Kastner M, Straus SE, McKibbon KA, Goldsmith CH (2009). "The capture-mark-recapture technique can be used as a stopping rule when searching in systematic reviews.". J Clin Epidemiol 62 (2): 149-57. DOI:10.1016/j.jclinepi.2008.06.001. PMID 18722088. Research Blogging.
- ↑ Spoor P, Airey M, Bennett C, Greensill J, Williams R (1996). "Use of the capture-recapture technique to evaluate the completeness of systematic literature searches.". BMJ 313 (7053): 342-3. PMID 8760743. PMC PMC2351754. [e]
- ↑ Egger M, Smith GD (1998). "Bias in location and selection of studies.". BMJ 316 (7124): 61-6. PMID 9451274. PMC PMC2665334.
- ↑ Cook DJ, Reeve BK, Guyatt GH, Heyland DK, Griffith LE, Buckingham L et al. (1996 Jan 24-31). "Stress ulcer prophylaxis in critically ill patients. Resolving discordant meta-analyses.". JAMA 275 (4): 308-14. PMID 8544272.
- ↑ Goodman SN (2002). "The mammography dilemma: a crisis for evidence-based medicine?". Ann Intern Med 137 (5 Part 1): 363-5. PMID 12204023.
- ↑ Slavin RE (January 1995). "Best evidence synthesis: an intelligent alternative to meta-analysis". J Clin Epidemiol 48 (1): 9–18. PMID 7853053. [e]
- ↑ Vale CL, Tierney JF, Burdett S (2013). "Can trial quality be reliably assessed from published reports of cancer trials: evaluation of risk of bias assessments in systematic reviews.". BMJ 346: f1798. DOI:10.1136/bmj.f1798. PMID 23610376. Research Blogging.
- ↑ Hartling L, Ospina M, Liang Y, Dryden DM, Hooton N, Krebs Seida J et al. (2009). "Risk of bias versus quality assessment of randomised controlled trials: cross sectional study.". BMJ 339: b4012. DOI:10.1136/bmj.b4012. PMID 19841007. PMC PMC2764034. Research Blogging.
- ↑ Higgins JPT, Green S (editors). Table 8.5.a: The Cochrane Collaboration's Tool for assessing risk of bias. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1 [updated September 2008]. The Cochrane Collaboration, 2008. Available from www.cochrane-handbook.org.
- ↑ Jadad AR, Moore RA, Carroll D, et al (1996). "Assessing the quality of reports of randomized clinical trials: is blinding necessary?". Control Clin Trials 17 (1): 1–12. DOI:10.1016/0197-2456(95)00134-4. PMID 8721797. Research Blogging.
- ↑ 35.0 35.1 35.2 Higgins JP, Thompson SG, Deeks JJ, Altman DG (September 2003). "Measuring inconsistency in meta-analyses". BMJ 327 (7414): 557–60. DOI:10.1136/bmj.327.7414.557. PMID 12958120. PMC 192859. Research Blogging.
- ↑ Higgins JP, Thompson SG (2002). "Quantifying heterogeneity in a meta-analysis.". Stat Med 21 (11): 1539-58. DOI:10.1002/sim.1186. PMID 12111919. Research Blogging.
- ↑ Fleiss JL (December 1986). "Analysis of data from multiclinic trials". Control Clin Trials 7 (4): 267–75. PMID 3802849. [e]
- ↑ Dickersin K, Berlin JA (1992). "Meta-analysis: state-of-the-science". Epidemiol Rev 14: 154–76. PMID 1289110. [e]
- ↑ Higgins JPT, Green S:Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Collaboration (2008). Retrieved on 2016-10-04.
- ↑ Ioannidis JP, Patsopoulos NA, Evangelou E (2007). "Uncertainty in heterogeneity estimates in meta-analyses.". BMJ 335 (7626): 914-6. DOI:10.1136/bmj.39343.408449.80. PMID 17974687. Research Blogging.
- ↑ Ioannidis JP (2008). "Interpretation of tests of heterogeneity and bias in meta-analysis.". J Eval Clin Pract 14 (5): 951-7. DOI:10.1111/j.1365-2753.2008.00986.x. PMID 19018930. Research Blogging.
- ↑ Altman DG, Bland JM (2003). "Interaction revisited: the difference between two estimates.". BMJ 326 (7382): 219. PMID 12543843. PMC PMC1125071.
- ↑ DerSimonian R, Laird N (1986). "Meta-analysis in clinical trials.". Control Clin Trials 7 (3): 177-88. DOI:10.1016/0197-2456(86)90046-2. PMID 3802833. Research Blogging.
- ↑ Riley RD, Higgins JP, Deeks JJ (2011). "Interpretation of random effects meta-analyses.". BMJ 342: d549. DOI:10.1136/bmj.d549. PMID 21310794. Research Blogging.
- ↑ 45.0 45.1 Diamond GA, Bax L, Kaul S (October 2007). "Uncertain effects of rosiglitazone on the risk for myocardial infarction and cardiovascular death". Ann. Intern. Med. 147 (8): 578–81. PMID 17679700. [e]
- ↑ Friedrich JO, Adhikari NK, Beyene J (2007). "Inclusion of zero total event trials in meta-analyses maintains analytic consistency and incorporates all available data.". BMC Med Res Methodol 7: 5. DOI:10.1186/1471-2288-7-5. PMID 17244367. PMC PMC1783664. Research Blogging.
- ↑ Bradburn MJ, Deeks JJ, Berlin JA, Russell Localio A (2007). "Much ado about nothing: a comparison of the performance of meta-analytical methods with rare events.". Stat Med 26 (1): 53-77. DOI:10.1002/sim.2528. PMID 16596572. Research Blogging.
- ↑ Sweeting MJ, Sutton AJ, Lambert PC (2004). "What to add to nothing? Use and avoidance of continuity corrections in meta-analysis of sparse data.". Stat Med 23 (9): 1351-75. DOI:10.1002/sim.1761. PMID 15116347. Research Blogging.
- ↑ 49.0 49.1 Cochrane Handbook. The standardized mean difference
- ↑ Cohen J (1992). "A power primer.". Psychol Bull 112 (1): 155-9. PMID 19565683. [e]
- ↑ Furukawa TA (1999). "From effect size into number needed to treat.". Lancet 353 (9165): 1680. DOI:10.1016/S0140-6736(99)01163-0. PMID 10335798. Research Blogging.
- ↑ Chinn S (2000). "A simple method for converting an odds ratio to effect size for use in meta-analysis.". Stat Med 19 (22): 3127-31. PMID 11113947. [e]
- ↑ Higgins JPT, Green S (editors). 9.6.2 What are subgroup analyses? in Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2 [updated September 2009]. The Cochrane Collaboration, 2009. Available from http:// www.cochrane-handbook.org.
- ↑ 54.0 54.1 54.2 Higgins JPT, Green S (editors). 9.6.3.1 Is the effect different in different subgroups? in Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2 [updated September 2009]. The Cochrane Collaboration, 2009. Available from http:// www.cochrane-handbook.org.
- ↑ 55.0 55.1 Higgins JP, Thompson SG (2004). "Controlling the risk of spurious findings from meta-regression.". Stat Med 23 (11): 1663-82. DOI:10.1002/sim.1752. PMID 15160401. Research Blogging.
- ↑ Bax L, Yu LM, Ikeda N, Moons KG (2007). "A systematic comparison of software dedicated to meta-analysis of causal studies.". BMC Med Res Methodol 7: 40. DOI:10.1186/1471-2288-7-40. PMID 17845719. PMC PMC2048970. Research Blogging.
- ↑ Wallace BC, Schmid CH, Lau J, Trikalinos TA (2009). "Meta-Analyst: software for meta-analysis of binary, continuous and diagnostic data.". BMC Med Res Methodol 9: 80. DOI:10.1186/1471-2288-9-80. PMID 19961608. PMC PMC2795760. Research Blogging.
- ↑ Cornell JE, Mulrow CD, Localio R, Stack CB, Meibohm AR, Guallar E, et al. Random-Effects Meta-analysis of Inconsistent Effects: A Time for Change. Ann Intern Med. 2014 Feb 18;160(4):267–70.
- ↑ Viechtbauer, W. (2010). Conducting meta-analyses in R with the metafor package. Journal of Statistical Software, 36(3), 1-48.
- ↑ Torsten Hothorn; Everitt, Brian. CRAN - Package HSAUR, 2nd ed.
- ↑ Gerstein HC, Miller ME, Byington RP, et al (June 2008). "Effects of intensive glucose lowering in type 2 diabetes". N. Engl. J. Med. 358 (24): 2545–59. DOI:10.1056/NEJMoa0802743. PMID 18539917. Research Blogging.
- ↑ Lau J, Antman EM, Jimenez-Silva J, Kupelnick B, Mosteller F, Chalmers TC (July 1992). "Cumulative meta-analysis of therapeutic trials for myocardial infarction". N. Engl. J. Med. 327 (4): 248–54. PMID 1614465. [e]
- ↑ Antman EM, Lau J, Kupelnick B, Mosteller F, Chalmers TC (July 1992). "A comparison of results of meta-analyses of randomized control trials and recommendations of clinical experts. Treatments for myocardial infarction". JAMA 268 (2): 240–8. PMID 1535110. [e]
- ↑ 64.0 64.1 Wetterslev J, Thorlund K, Brok J, Gluud C (2008). "Trial sequential analysis may establish when firm evidence is reached in cumulative meta-analysis.". J Clin Epidemiol 61 (1): 64-75. DOI:10.1016/j.jclinepi.2007.03.013. PMID 18083463. Research Blogging.
- ↑ Thorlund K, Imberger G, Walsh M, Chu R, Gluud C, Wetterslev J et al. (2011). "The number of patients and events required to limit the risk of overestimation of intervention effects in meta-analysis-a simulation study.". PLoS One 6 (10): e25491. DOI:10.1371/journal.pone.0025491. PMID 22028777. PMC PMC3196500. Research Blogging.
- ↑ Brok J, Thorlund K, Gluud C, Wetterslev J (2008). "Trial sequential analysis reveals insufficient information size and potentially false positive results in many meta-analyses.". J Clin Epidemiol 61 (8): 763-9. DOI:10.1016/j.jclinepi.2007.10.007. PMID 18411040. Research Blogging.
- ↑ Sud S, Douketis J (July 2009). "ACP Journal Club. The devil is in the details...or not? A primer on individual patient data meta-analysis". Ann. Intern. Med. 151 (2): JC1–2, JC1–3. PMID 19620147. [e]
- ↑ Riley RD, Lambert PC, Abo-Zaid G (2010). "Meta-analysis of individual participant data: rationale, conduct, and reporting.". BMJ 340: c221. DOI:10.1136/bmj.c221. PMID 20139215. Research Blogging.
- ↑ 69.0 69.1 Poynard T, Munteanu M, Ratziu V, et al (June 2002). "Truth survival in clinical research: an evidence-based requiem?". Ann. Intern. Med. 136 (12): 888–95. PMID 12069563. [e]
- ↑ Elliott JH, Turner T, Clavisi O, Thomas J, Higgins JP, Mavergames C et al. (2014). "Living systematic reviews: an emerging opportunity to narrow the evidence-practice gap.". PLoS Med 11 (2): e1001603. DOI:10.1371/journal.pmed.1001603. PMID 24558353. PMC PMC3928029. Research Blogging.
- ↑ Grewal S, Klassen TP (2014). "The tale of 2 trials: disentangling contradictory evidence on hypertonic saline for acute bronchiolitis.". JAMA Pediatr 168 (7): 607-9. DOI:10.1001/jamapediatrics.2014.423. PMID 24862208. Research Blogging.
- ↑ Thompson SG, Sharp SJ (1999). "Explaining heterogeneity in meta-analysis: a comparison of methods.". Stat Med 18 (20): 2693-708. PMID 10521860.
- ↑ Thompson SG, Higgins JP (2002). "How should meta-regression analyses be undertaken and interpreted?". Stat Med 21 (11): 1559-73. DOI:10.1002/sim.1187. PMID 12111920. Research Blogging.
- ↑ van Houwelingen HC, Arends LR, Stijnen T (2002). "Advanced methods in meta-analysis: multivariate approach and meta-regression.". Stat Med 21 (4): 589-624. PMID 11836738.
- ↑ Jackson D, White IR, Thompson SG (2009). "Extending DerSimonian and Laird's methodology to perform multivariate random effects meta-analyses.". Stat Med. DOI:10.1002/sim.3602. PMID 19408255. Research Blogging.
- ↑ Farnett L, Mulrow CD, Linn WD, Lucey CR, Tuley MR (1991). "The J-curve phenomenon and the treatment of hypertension. Is there a point beyond which pressure reduction is dangerous?". JAMA 265 (4): 489-95. DOI:10.1001/jama.1991.03460040065031. PMID 1824642. Research Blogging.
- ↑ Lumley,T. (2009) rmeta: Meta-analysis
- ↑ Everitt B, Hothorn, T. (2009) HSAUR2
- ↑ Everitt B, Hothorn, T.. A Handbook of Statistical Analyses Using R, Second Edition. Chapman Hall/CRC. ISBN 1-4200-7933-6.
- ↑ Lambert PC, Sutton AJ, Abrams KR, Jones DR (2002). "A comparison of summary patient-level covariates in meta-regression with individual patient data meta-analysis.". J Clin Epidemiol 55 (1): 86-94. PMID 11781126. [e]
- ↑ Simmonds MC, Higgins JP (2007). "Covariate heterogeneity in meta-analysis: criteria for deciding between meta-regression and individual patient data.". Stat Med 26 (15): 2982-99. DOI:10.1002/sim.2768. PMID 17195960. Research Blogging.
- ↑ Lumley T (August 2002). "Network meta-analysis for indirect treatment comparisons". Stat Med 21 (16): 2313–24. DOI:10.1002/sim.1201. PMID 12210616. Research Blogging.
- ↑ Lu G, Ades AE. Combination of direct and indirect evidence in mixed treatment comparisons. Stat Med. 2004 Oct 30;23(20):3105-24. PMID 15449338
- ↑ Salanti G, Kavvoura FK, Ioannidis JP (April 2008). "Exploring the geometry of treatment networks". Ann. Intern. Med. 148 (7): 544–53. PMID 18378949. [e]
- ↑ Ioannidis JP (2009). "Integration of evidence from multiple meta-analyses: a primer on umbrella reviews, treatment networks and multiple treatments meta-analyses.". CMAJ 181 (8): 488-93. DOI:10.1503/cmaj.081086. PMID 19654195. PMC PMC2761440. Research Blogging.
- ↑ Song F, Loke YK, Walsh T, Glenny AM, Eastwood AJ, Altman DG (2009). "Methodological problems in the use of indirect comparisons for evaluating healthcare interventions: survey of published systematic reviews". BMJ 338: b1147. PMID 19346285. PMC 2665205. [e]
- ↑ Kent DM, Thaler DE (September 2008). "Stroke prevention--insights from incoherence". N. Engl. J. Med. 359 (12): 1287–9. DOI:10.1056/NEJMe0806806. PMID 18753641. Research Blogging.
- ↑ Thijs V, Lemmens R, Fieuws S. Network meta-analysis: simultaneous meta-analysis of common antiplatelet regimens after transient ischaemic attack or stroke. ur Heart J. 2008 May;29(9):1086-92. Epub 2008 Mar 17. PMID 18349026
- ↑ Chou, Roger; Susan Carson, Benjamin Chan (2009-02-01). "Gabapentin Versus Tricyclic Antidepressants for Diabetic Neuropathy and Post-Herpetic Neuralgia: Discrepancies Between Direct and Indirect Meta-Analyses of Randomized Controlled Trials". Journal of General Internal Medicine 24 (2): 178-188. DOI:10.1007/s11606-008-0877-5. PMID 19089502. Retrieved on 2009-01-26. Research Blogging.
- ↑ Takwoingi, Yemisi; Mariska M.G. Leeflang, Jonathan J. Deeks (2013-04-02). "Empirical Evidence of the Importance of Comparative Studies of Diagnostic Test Accuracy". Annals of Internal Medicine 158 (7): 544-554. DOI:10.7326/0003-4819-158-7-201304020-00006. ISSN 0003-4819. Retrieved on 2013-04-01. Research Blogging.
- ↑ Singh JA, Christensen R, Wells GA, Suarez-Almazor ME, Buchbinder R, Lopez-Olivo MA et al. (2009). "A network meta-analysis of randomized controlled trials of biologics for rheumatoid arthritis: a Cochrane overview.". CMAJ 181 (11): 787-96. DOI:10.1503/cmaj.091391. PMID 19884297. PMC PMC2780484. Research Blogging.
- ↑ Singh JA, Christensen R, Wells GA, Suarez-Almazor ME, Buchbinder R, Lopez-Olivo MA et al. (2009). "Biologics for rheumatoid arthritis: an overview of Cochrane reviews.". Cochrane Database Syst Rev (4): CD007848. DOI:10.1002/14651858.CD007848.pub2. PMID 19821440. Research Blogging.
- ↑ Diagnostic Test Accuracy Working Group (2009) Handbook for DTA Reviews. Cochrane Collaboration
- ↑ 94.0 94.1 Leeflang MM, Deeks JJ, Gatsonis C, Bossuyt PM, Cochrane Diagnostic Test Accuracy Working Group (2008). "Systematic reviews of diagnostic test accuracy.". Ann Intern Med 149 (12): 889-97. PMID 19075208 pmcid=.
- ↑ Toft N, Nielsen SS (2009). "Summary receiver operating characteristics (SROC) and hierarchical SROC models for analysis of diagnostic test evaluations of antibody ELISAs for paratuberculosis.". Prev Vet Med 92 (3): 249-55. DOI:10.1016/j.prevetmed.2009.08.019. PMID 19747743. Research Blogging.
- ↑ 96.0 96.1 Harbord RM, Deeks JJ, Egger M, Whiting P, Sterne JA (2007). "A unification of models for meta-analysis of diagnostic accuracy studies.". Biostatistics 8 (2): 239-51. DOI:10.1093/biostatistics/kxl004. PMID 16698768. Research Blogging.
- ↑ Gatsonis C, Paliwal P (2006). "Meta-analysis of diagnostic and screening test accuracy evaluations: methodologic primer.". AJR Am J Roentgenol 187 (2): 271-81. DOI:10.2214/AJR.06.0226. PMID 16861527. Research Blogging.
- ↑ Bruening W, Fontanarosa J, Tipton K, Treadwell JR, Launders J, Schoelles K (2010). "Systematic review: comparative effectiveness of core-needle and open surgical biopsy to diagnose breast lesions.". Ann Intern Med 152 (4): 238-46. DOI:10.1059/0003-4819-152-1-201001050-00190. PMID 20008742. Research Blogging.
- ↑ Avni T, Mansur N, Leibovici L, Paul M (2010). "PCR using blood for diagnosis of invasive pneumococcal disease: systematic review and meta-analysis.". J Clin Microbiol 48 (2): 489-96. DOI:10.1128/JCM.01636-09. PMID 20007385. PMC PMC2815606. Research Blogging.
- ↑ 100.0 100.1 Whitlock EP, Lin JS, Chou R, Shekelle P, Robinson KA (2008). "Using existing systematic reviews in complex systematic reviews.". Ann Intern Med 148 (10): 776-82. PMID 18490690. [e]
- ↑ Becker LA, Oxman AD. Chapter 22: Overviews of reviews In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Inervenstions. Version 5.0.1 (updated September, 2008). Available from www.cochrane-handbook.org
- ↑ Oxman AD, Guyatt GH (1991). "Validation of an index of the quality of review articles.". J Clin Epidemiol 44 (11): 1271-8. PMID 1834807. [e]
- ↑ Shea BJ, Grimshaw JM, Wells GA, Boers M, Andersson N, Hamel C et al. (2007). "Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews.". BMC Med Res Methodol 7: 10. DOI:10.1186/1471-2288-7-10. PMID 17302989. PMC PMC1810543. Research Blogging.
- ↑ Olsen O, Middleton P, Ezzo J, et al (2001). "Quality of Cochrane reviews: assessment of sample from 1998". BMJ 323 (7317): 829–32. PMID 11597965. [e]
- ↑ Yank V, Rennie D, Bero LA (2007). "Financial ties and concordance between results and conclusions in meta-analyses: retrospective cohort study.". BMJ 335 (7631): 1202-5. DOI:10.1136/bmj.39376.447211.BE. PMID 18024482. PMC PMC2128658. Research Blogging.
- ↑ Dechartres A, Trinquart L, Boutron I, Ravaud P (2013). "Influence of trial sample size on treatment effect estimates: meta-epidemiological study.". BMJ 346: f2304. DOI:10.1136/bmj.f2304. PMID 23616031. PMC PMC3634626. Research Blogging.
- ↑ Nüesch E, Trelle S, Reichenbach S, Rutjes AW, Tschannen B, Altman DG et al. (2010). "Small study effects in meta-analyses of osteoarthritis trials: meta-epidemiological study.". BMJ 341: c3515. DOI:10.1136/bmj.c3515. PMID 20639294. PMC PMC2905513. Research Blogging.
- ↑ Sterne JA, Egger M, Smith GD (2001). "Systematic reviews in health care: Investigating and dealing with publication and other biases in meta-analysis.". BMJ 323 (7304): 101-5. PMID 11451790. PMC PMC1120714.
- ↑ F. Richy, O. Ethgen, O. Bruyere, F. Deceulaer & J. Reginster : From Sample Size to Effect-Size: Small Study Effect Investigation (SSEi) . The Internet Journal of Epidemiology. 2004 Volume 1 Number 2
- ↑ Sutton AJ, Duval SJ, Tweedie RL, Abrams KR, Jones DR (2000). "Empirical assessment of effect of publication bias on meta-analyses.". BMJ 320 (7249): 1574-7. PMID 10845965. PMC PMC27401.
- ↑ 111.0 111.1 111.2 Egger M, Davey Smith G, Schneider M, Minder C (1997). "Bias in meta-analysis detected by a simple, graphical test.". BMJ 315 (7109): 629-34. PMID 9310563. PMC PMC2127453.
- ↑ Pham B, Platt R, McAuley L, Klassen TP, Moher D (2001). "Is there a "best" way to detect and minimize publication bias? An empirical evaluation.". Eval Health Prof 24 (2): 109-25. PMID 11523382.
- ↑ Sterne, JAC, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials BMJ 2011; 343:d4002 DOI:10.1136/bmj.d4002
- ↑ Terrin N et al. (2005). "In an empirical evaluation of the funnel plot, researchers could not visually identify publication bias". J Clin Epidemiol 58: 894–901. DOI:10.1016/j.jclinepi.2005.01.006. PMID 16085192. Research Blogging.
- ↑ Furukawa TA, Watanabe N, Omori IM, Montori VM, Guyatt GH (February 2007). "Association between unreported outcomes and effect size estimates in Cochrane meta-analyses". JAMA 297 (5): 468–70. DOI:10.1001/jama.297.5.468-b. PMID 17284696. Research Blogging.
- ↑ Chan AW, Hróbjartsson A, Haahr MT, Gøtzsche PC, Altman DG (May 2004). "Empirical evidence for selective reporting of outcomes in randomized trials: comparison of protocols to published articles". JAMA 291 (20): 2457–65. DOI:10.1001/jama.291.20.2457. PMID 15161896. Research Blogging.
- ↑ Contopoulos-Ioannidis DG, Gilbody SM, Trikalinos TA, Churchill R, Wahlbeck K, Ioannidis JP (2005). "Comparison of large versus smaller randomized trials for mental health-related interventions.". Am J Psychiatry 162 (3): 578-84. DOI:10.1176/appi.ajp.162.3.578. PMID 15741476. Research Blogging.
- ↑ Villar J, Piaggio G, Carroli G, Donner A (1997). "Factors affecting the comparability of meta-analyses and largest trials results in perinatology.". J Clin Epidemiol 50 (9): 997-1002. PMID 9363033. [e]
- ↑ Ioannidis JP, Cappelleri JC, Lau J (1998). "Meta-analyses and large randomized, controlled trials.". N Engl J Med 338 (1): 59; author reply 61-2. DOI:10.1056/NEJM199801013380112. PMID 9424563. Research Blogging.
- ↑ Roseman, Michelle; Katherine Milette, Lisa A. Bero, James C. Coyne, Joel Lexchin, Erick H. Turner, Brett D. Thombs (2011-03-09). "Reporting of Conflicts of Interest in Meta-analyses of Trials of Pharmacological Treatments". JAMA: The Journal of the American Medical Association 305 (10): 1008 -1017. DOI:10.1001/jama.2011.257. Retrieved on 2011-03-10. Research Blogging.
- ↑ Shojania KG, Sampson M, Ansari MT, Ji J, Doucette S, Moher D (August 2007). "How quickly do systematic reviews go out of date? A survival analysis". Ann. Intern. Med. 147 (4): 224–33. PMID 17638714. [e]
- ↑ The use of older studies in meta-analyses of medical interventions: a survey (Text.Serial.Journal) (2009-05-11). Retrieved on 2009-06-04.
- ↑ Takwoingi Y, Hopewell S, Tovey D, Sutton AJ (2013). "A multicomponent decision tool for prioritising the updating of systematic reviews.". BMJ 347: f7191. DOI:10.1136/bmj.f7191. PMID 24336453. Research Blogging.
- ↑ Sampson M, Shojania KG, McGowan J, et al (August 2008). "Surveillance search techniques identified the need to update systematic reviews". J Clin Epidemiol 61 (8): 755–62. DOI:10.1016/j.jclinepi.2007.10.003. PMID 18586179. Research Blogging.
- ↑ Ioannidis J, Lau J (2001). "Evolution of treatment effects over time: empirical insight from recursive cumulative metaanalyses.". Proc Natl Acad Sci U S A 98 (3): 831-6. DOI:10.1073/pnas.021529998. PMID 11158556. PMC PMC14669. Research Blogging.
- ↑ Siontis KC, Hernandez-Boussard T, Ioannidis JP (2013). "Overlapping meta-analyses on the same topic: survey of published studies.". BMJ 347: f4501. DOI:10.1136/bmj.f4501. PMID 23873947. PMC PMC3716360. Research Blogging.
 KSF
KSF