Metabolism
 From Citizendium - Reading time: 8 min
From Citizendium - Reading time: 8 min
Metabolism (from Greek μεταβολισμός "metabolismos") is the biochemical modification of chemical compounds by living organisms and cells. In common usage, the word is often used to refer to the basal metabolic rate, the "set point" that each person has in breaking down food energy and building up their own body. In multicellular creatures like humans, its meaning encompasses the overall ingestion of food and excretion of wastes, as well as the building up of muscles and the growth of the body. In terms of the whole organism, metabolism includes the chemical conversion of ingested items other than food, like drugs and poisons (see Drug metabolism). This article describes the actual biology of metabolism at a cellular level, which explains just how those processes are carried out.
Metabolism includes: (1) anabolism, in which a cell uses chemical energy and reducing power to construct complex molecules, and perform life functions such as creating cellular structure; and (2) catabolism, in which a cell breaks down complex molecules to yield the chemical energy and reducing power. Cell metabolism involves complex sequences of controlled chemical reactions called metabolic pathways. Just as the word metabolism can be used to describe processes in a whole organism, the terms "anabolism" and "catabolism" can similarly be used in this way. For example, anabolic processes can also refer to building up muscle and adding body weight, while catabolic processes can refer to the loss of muscle mass and body fat.

History[edit]

The first controlled experiments on human metabolism were published by Santorio Santorio in 1614 in his book Ars de statica medecina, in which he described experiments in which he weighed himself in a chair suspended from a steelyard balance (see image), before and after eating, sleeping, working, sex, fasting, depriving from drinking, and excreting. He found that by far the greatest part of the food he took in was lost from the body through perspiratio insensibilis (insensible perspiration). In medicine and the health sciences, the term "insensible losses" is still used to refer to fluids that escape the body without leaving easily-measurable traces behind.
At about the same time, Jan Baptist van Helmont made the first observations regarding photosynthesis, when he discovered that growing plants drew almost no matter from the surrounding soil. The physical source of the plant's growth was not obvious until later experiments, which delved into the process now known as photosynthesis.
In the 18th century, Joseph Priestley discovered that green plants released a substance (later found to be oxygen) that could sustain the life of a mouse in an enclosed chamber. Jan Ingenhousz extended Priestley's experiments to show that oxygen was produced when light was cast on the plant, while Jean Senebier showed that carbon dioxide was absorbed by plants during photosynthesis. In 1804, Nicolas de Saussure discovered that plant growth was the result of both the fixation of atmospheric carbon dioxide () into the plant, and the incorporation of water.
Between 1854 and 1864, Louis Pasteur discovered that glucose fermentation is due to microorganisms, and, in 1897, Eduard Buchner proved that cell-free yeast extracts could also perform these reactions, and so the ability to ferment was not limited to entire living creatures (cells)- but included certain portions of their physical contents. Subsequent investigations showed that living organisms, with few exceptions, metabolize glucose using the same mechanism, namely, by a biochemical pathway that breaks down sugar.
Overview: Harnessing energy and making chemical bonds[edit]
Living things, like all things, obey the laws of thermodynamics. That means that energy and matter cannot be created from nothing; cool things always get colder rather than warmer, and each fragment of a whole are smaller than the whole itself. But, unlike inanimate things, cells and tissues are able to harness energy and matter to change in ways that give the illusion of defying those laws. A baby does grow. A walrus' body is warmer than its icy surroundings. An amoeba can divide and shortly be two amoebas, each one the same size of the original cell that split. The metabolism of the baby, the walrus, and the amoeba is responsible for all these processes. Of course, rather than defy the laws of thermodynamics, the chemical reactions that make up metabolic processes always obey them.
Enzymes present in cells can catalyze a large variety of chemical reactions with exquisite specificity. Generally, enzymes are protein molecules that make reactions go faster by bringing the reactant molecules close together in just the right orientation for a chemical change to occur. Sometimes these enzymes are floating free in the cytoplasm of the cell, other times they are corralled together within a compartment of the cell, a special organelle. For example, the mitochondrion of cells contains enzymes for oxidative phosphorylation (a catabolic process). The Golgi apparatus of cells contains many of the enzymes used for protein posttranslational modification (an anabolic process).
Often, the chemical reactions needed to synthesize useful cell components require energy. Chemists describe these reactions as involving a positive change in free energy. Such chemical transformations are not spontaneous, but "uphill", requiring more than just the mixing of the substrates. In these cases, specific enzymes may couple each "uphill" (non-spontaneous or energy requiring) reaction to a second, steep "downhill" (very spontaneous or energy releasing) reaction. Thus, thermodynamically favorable reactions can be used to "drive" each thermodynamically unfavorable one - such that the the overall process goes on its own, as a spontaneous series of reactions.
ATP: the energy currency of cells[edit]
There is one particular energetically favourable reaction that is repeatedly used to drive "uphill" reactions in metabolism:
- Adenosine triphosphate + water → Adenosine diphosphate + phosphate ion + hydrogen ion
This reaction, the hydrolysis of Adenosine triphosphate (ATP) into Adenosine diphosphate and two ions, occurs often in metabolic pathways. ATP is sometimes called the "energy currency" of cells because it is used to "finance" these uphill reactions. To regenerate it for further reactions a large amount of energy is needed to recombine the products shown in the equation above. This is done by coupling the uphill synthesis of ATP to other energy-releasing reactions and is so ubiquitous that organisms can be classified according to how they derive energy for the process. Organisms can be classified as either Phototrophic or Chemotrophic.
Phototrophic[edit]
Phototrophic organisms can obtain energy from light. In these reactions, excitation of a photosynthetic reaction centre is caused by the absorption of a light photon. During the process, the reaction center loses an electron that excites (reduces) an electron acceptor, such as pheophytin, initiating a flow of electrons down an electron transport chain present in the thylakoid membrane. The energy released in the electron transfer steps serves to create a proton gradient across the membrane; its dissipation is used by ATP synthase as the energy to synthesise ATP from ADP and a phosphate anion by photophosphorylation (see Chemiosmotic hypothesis). Depending on the organism, the reaction center regains the lost electron by either recycling the excited electrons or taking one from an electron donor. In plants, a water molecule serves as the electron donor through a process called photolysis, that releases oxygen gas as a waste product.
Chemotrophic[edit]
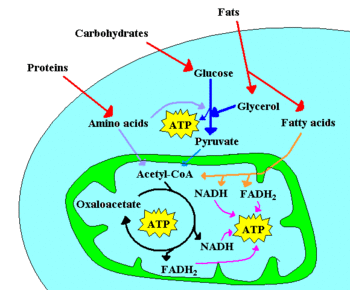
Chemotrophic organisms obtain energy from chemical reactions. For example, glucose can be oxidized to pyruvate through glycolysis. This yields two molecules of ATP for each molecule of glucose, by substrate-level phosphorylation, and four electrons, which reduce two NAD+ molecules to NADH.
For glycolysis to continue, the NADH must be recycled to NAD+ by donating the electrons to an electron acceptor. Respiration is said to occur if this electron acceptor is external to the metabolism, and may be either anaerobic or aerobic. Fermentation, on the other hand, does not use an external electron acceptor: in this case, the electron acceptor is a product of glycolysis, usually pyruvate or a pyruvate derivative.
Acetyl-CoA is a pivotal molecule during aerobic respiration. Acetyl-CoA is derived from pyruvate, but can also be formed through β-oxidation of fatty acids or through the catabolism of amino acids, and is oxidized to CO2 through the Krebs cycle. The Krebs cycle releases eight electrons from each acetyl-CoA molecule, which are eventually used in aerobic organisms to reduce oxygen (terminal electron acceptor) via an electron transport chain. This is part of the process to synthesis more ATP, known as oxidative phosphorylation, and is very similar to photophosphorylation in phototrophs.
Reducing Power: obtaining electrons for chemical bonds[edit]
Reducing power is an important input into many anabolic pathways, including the Calvin cycle of photosynthesis, the biosynthesis of amino acids, and the biosynthesis of fatty acids. Reducing power is usually supplied as hydrogen equivalents carried by NADPH. Organisms can be classified according to the primary source of this reducing power as:
Organotrophic[edit]
These organisms use organic compounds (e.g. glucose) as the primary electron source.
Lithotrophic[edit]
These organisms use inorganic compounds (e.g. Fe2+, (iron ions)) as primary electron source.
Regulation of metabolism in animals[edit]
In animals, metabolism is controlled by the endocrine system through the secretion of hormones. Some hormones have anabolic actions on the body, others have mainly catabolic actions. For example, testosterone is an anabolic hormone, and synthetic steroids that produce the anabolic actions are known as anabolic steroids. Cortisol on the other hand, which is a steroid hormone produced by the adrenal gland, is a catabolic hormone.
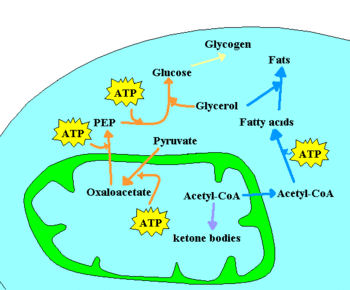
Two hormones synthesized by the pancreas, insulin and glucagon, are particularly important. Insulin is secreted when blood glucose levels are high, and it stimulates glucose uptake by muscle, glycogen synthesis, and triacylglyceride synthesis by adipose tissue (fat). It also inhibits gluconeogenesis and glycogen degradation. Glucagon is secreted when blood glucose levels are low, and its effects are opposite to those of insulin. In the liver, glucagon stimulates glycogen degradation and the absorption of gluconeogenic aminoacids, and it inhibits glycogen synthesis and promotes the release of fatty acids by adipose tissue.
In mammals and other warm blooded animals, many metabolic process are ultimately controlled by the central nervous system, which regulates the endocrine system. The central nervous system and endocrine system are influenced by the balance between the energy demands of the organism, and its energy stores (see also Hunger). For example, fat stores secrete a hormone called leptin that acts at the hypothalamus to regulate hormone secretion. The hypothalamus is also sensitive to circulating concentrations of glucose and insulin, and to body temperature. When the ambient temperature is low, the metabolic rate of an endothermic animal will increase in order to generate more body heat (thermogenesis). In animals that hibernate, the body temperature drops down enough that the basal metabolic rate is quite low, conserving energy over a winter period of inactivity.
Some ectothermic animals, like reptiles, regulate their body temperature by their behavior. These "cold blooded" creatures, including lizards, snakes, and turtles, keep at an optimum body temperature by heating up in the sun (basking) and cooling down in the shade or the cool earth of a burrow. The metabolism of these animals also changes with body temperature, and explains the sluggish movements of an ectotherm in colder seasons or times of day.
 KSF
KSF