Molecule
 From Citizendium - Reading time: 5 min
From Citizendium - Reading time: 5 min
In chemistry, a molecule is an aggregate of two or more atoms in a definite arrangement held together by chemical bonds. [1] [2] [3] [4] [5] Chemical substances are not infinitely divisible into smaller fractions of the same substance: a molecule is generally considered the smallest particle of a pure substance that still retains its composition and chemical properties.[6] Certain pure substances (e.g., metals, molten salts, crystals, etc.) are best understood as being composed of networks or aggregates of atoms or ions instead of molecular units.

In the molecular sciences, a molecule is a sufficiently stable, electrically neutral entity composed of two or more atoms.[7]
The concept of a monatomic molecule, i.e. a single-atom molecule as found in noble gases, is used almost exclusively in the kinetic theory of gases, where the fundamental gas particles are conventionally termed "molecules" regardless of their composition.[8]
History[edit]
Although the concept of molecules was first introduced in 1811 by Avogadro, and was accepted by many chemists as a result of Dalton's laws of Definite and Multiple Proportions (1803-1808), the existence of molecules as anything other than convenient mathematical constructs was still an open debate in the 19th century physics community—with as notable exceptions Boltzmann, Maxwell, Gibbs, and Van der Waals. The concept was strenuously resisted by early positivists such as Mach. The very different measurements of Avogadro's number, which all produced essentially the same value, finally gave the definite proof of the existence of molecules. The modern theory of molecules makes great use of the many numerical techniques offered by computational chemistry. Dozens of molecules have now been identified in interstellar space by microwave spectroscopy.
Overview[edit]
The science of molecules is called molecular chemistry or molecular physics, depending on the focus. Molecular chemistry deals with the laws governing the interaction between molecules that results in the formation and breakage of chemical bonds, while molecular physics deals with the laws governing their structure and properties. In practice, however, this distinction is vague. In molecular sciences, a molecule consists of a stable system (bound state) comprising two or more atoms. Polyatomic ions may sometimes be usefully thought of as electrically-charged molecules. The term unstable molecule is used for very reactive species, i.e., short-lived assemblies (resonances) of electrons and nuclei, such as radicals, molecular ions, Rydberg molecules, transition states, Van der Waals complexes, or systems of colliding atoms as in Bose-Einstein condensates.
A peculiar use of the term molecular is as a synonym to covalent, which arises from the fact that, unlike molecular covalent compounds, ionic compounds do not yield well-defined smallest particles that would be consistent with the definition above. However, the same problem also arises for some (but not all) covalent compounds. No typical "smallest particle" can be defined for covalent crystals, or network solids, which are composed of repeating unit cells that extend indefinitely either in a plane (such as in graphite) or three-dimensionally (such as in diamond).
While all gases exist as molecules by definition (as the term for gas particles), not all solids and liquids do. In fact, many of the most familiar substances in ordinary experience, such as rocks, crystals, and metals, are composed of atoms or ions, but are not made of molecules.
Chemical bond[edit]
- See main article chemical bond
In a molecule, the atoms are joined by shared pairs of electrons in a chemical bond. It may consist of atoms of the same chemical element, as with oxygen (O2), or of different elements, as with water (H2O).
Size[edit]
Most molecules are far too small to be seen with the naked eye, but there are exceptions. DNA, a macromolecule, can reach macroscopic sizes. The smallest molecule is the hydrogen molecule. The interatomic distance is 74 picometres (0.74 Å). But the size of its electron cloud is difficult to define precisely. Typical molecules have a dimension of a few to several dozen Å.
Empirical formula[edit]
- See main article empirical formula
The empirical formula of a molecule is the simplest integer ratio of the chemical elements that constitute the compound. For example, water (H2O) is composed of a 2:1 ratio of hydrogen to oxygen, and ethyl alcohol or ethanol (CH3CH2OH) is always composed of carbon, hydrogen, and oxygen in a 2:6:1 ratio, i.e., its empirical formula is C2H6O. However, this does not determine the kind of molecule uniquely - dimethyl ether (CH3OCH3), for instance, has the same ratios as ethanol, and hence the same empirical formula. Molecules with the same atoms in different arrangements are called isomers. The empirical formula contains often the same number of atoms as the molecular formula but not always. For example the molecule acetylene has molecular formula C2H2 (sometimes written as HCCH), but the simplest integer ratio of elements is CH, which is the empirical formula of the acetylene molecule.
Chemical formula[edit]
- See main article chemical formula
The chemical formula reflects the exact number of atoms that compose a molecule.
Molecular mass[edit]
- See main article molecular mass
The molecular mass can be calculated from the chemical formula and is expressed in conventional units equal to 1/12 from the mass of a 12C isotope atom. For network solids, the term formula unit is used in stoichiometric calculations.
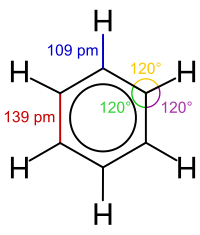
Molecular geometry[edit]
Molecules have fixed equilibrium geometries—bond lengths and angles—about which they continuously oscillate through vibrational and rotational motions. A pure substance is composed of molecules with the same average geometrical structure. The chemical formula and the structure of a molecule are the two important factors that determine its properties, particularly its reactivity. Isomers share a chemical formula but normally have very different properties because of their different structures. Stereoisomers, a particular type of isomers, may have very similar physico-chemical properties and at the same time very different biochemical activities.
The triatomic molecules that form carbon dioxide (CO2) and water (H2O) have different geometries. The three atoms in CO2 are collinear, with the carbon atom between the oxygen atoms, while the atoms in H2O form a 104.5 degree angle with the oxygen atom at the vertex.

Smaller molecules (up to about twenty or thirty atoms) can often be seen as rigid rotors of different shapes (linear, spherical, symmetric, and asymmetric) see classification of rigid rotors.
Molecular spectroscopy[edit]
Molecular spectroscopy deals with the response (spectrum) of molecules interacting with probing signals of known energy (or frequency, according to Planck's formula). Scattering theory provides the theoretical background for spectroscopy.
The probing signal used in spectroscopy can be an electromagnetic wave or a beam of particles (electrons, positrons, etc.) The molecular response can consist of signal absorption (absorption spectroscopy), the emission of another signal (emission spectroscopy), fragmentation, or chemical changes.
Spectroscopy is recognized as a powerful tool in investigating the microscopic properties of molecules, in particular their energy levels. In order to extract maximum microscopic information from experimental results, spectroscopy is often coupled with chemical computations.
Attribution[edit]
- Some content on this page may previously have appeared on Wikipedia.
Footnotes[edit]
- ↑ Pauling, Linus (1970). General Chemistry. New York: Dover Publications, Inc.. ISBN 0486656224.
- ↑ Ebbin, Darrell, D. (1990). General Chemistry, 3th Ed.. Boston: Houghton Mifflin Co.. ISBN 0395433029.
- ↑ Brown, T.L. (2003). Chemistry – the Central Science, 9th Ed.. New Jersey: Prentice Hall. ISBN 0130669970.
- ↑ Chang, Raymond (1998). Chemistry, 6th Ed.. New York: McGraw Hill. ISBN 0-07-115221-0.
- ↑ Zumdahl, Steven S. (1997). Chemistry, 4th ed.. Boston: Houghton Mifflin. ISBN 0-669-41794-7.
- ↑ Molecule Definition - Frostburg State University (Department of Chemistry)
- ↑ Definition of Molecule - IUPAC
- ↑ [1] [2] [3]
 KSF
KSF