Organic chemistry
 From Citizendium - Reading time: 11 min
From Citizendium - Reading time: 11 min
Organic chemistry is a specific discipline within the subject of chemistry. It is the scientific study of the structure, properties, composition, reactions, and preparation (by synthesis or by other means) of chemical compounds of carbon and hydrogen, which may contain any number of other elements, such as nitrogen, oxygen, halogens, and more rarely phosphorus or sulphur [1] [2].
The original definition of organic chemistry came from the misperception that these compounds were always related to life processes, but now it is known that life also depends heavily on inorganic chemistry; for example, many enzymes rely on transition metals such as iron and copper; and materials such as shells, teeth, and bones are part organic, part inorganic in composition. Inorganic chemistry deals, apart from elemental carbon, only with simple carbon compounds, with molecular structures which do not contain carbon to carbon connections (its oxides, acids, salts, carbides, and minerals). This does not mean that single-carbon organic compounds do not exist (viz. methane and its simple derivatives). Compounds that are related to life processes are dealt with in the branch of chemistry which is called biochemistry.
Because of their unique properties, multi-carbon compounds exhibit extremely large variety and the range of application of organic compounds is enormous. They form the basis of or are important constituents of many products (paints, plastics, food, explosives, drugs, petrochemicals, and many others) and of course (apart from a very few exceptions) they form the basis of all life processes.
The different shapes and chemical reactivities of organic molecules provide an astonishing variety of functions, like those of enzyme catalysts in biochemical reactions of live systems. The autopropagating nature of these is what life is all about.
Because of the special properties of carbon, it is likely that life on other star systems will be found to be carbon-based, in spite of speculations about the possibility of substituting silicon, which lies just below carbon in the periodic table.
Trends in organic chemistry include chiral synthesis, green chemistry, microwave chemistry, fullerenes and microwave spectroscopy.
Historic highlights[edit]

Towards the beginning of the nineteenth century, chemists generally thought that compounds from living organisms were too complicated in structure and that these compounds, through a 'vital force' or vitalism, were unique in that they could self-propagate. They named these compounds 'organic' and proceeded to ignore them.
Organic chemistry received a boost when it was realized that these compounds could be treated in ways similar to inorganic compounds and could be manufactured by means other than 'vital force'. Around 1816[Michel Chevreuil started a study of soaps made from various fats and alkali. He separated the different acids that, in combination with the alkali, produced the soap. Since these were all individual compounds, he demonstrated that it was possible to make a chemical change in various fats (which traditionally come from organic sources), producing new compounds, without 'vital force'.
The real event that has completely destroyed the myth of vitalism occurred, however, when in 1828 Friedrich Wöhler first manufactured the organic chemical urea (carbamide), a constituent of the liquid waste matter urine from the inorganic ammonium cyanate, NH4OCN, in what is now called the Wöhler synthesis.
A great next step was when in 1856 William Henry Perkin, while trying to manufacture quinine, again accidentally came to manufacture the organic dye now called Perkin's mauve, which by generating a huge amount of money greatly increased interest in organic chemistry. Another step was the laboratory preparation of DDT by Othmer Zeidler in 1874, but the insecticide properties of this compound were not discovered till much later.
The history of organic chemistry continues with the discovery of petroleum and its separation into fractions according to boiling ranges. The conversion of different compound types or individual compounds by various chemical processes created the petroleum chemistry leading to the birth of the petrochemical industry, which successfully manufactured artificial rubbers, the various organic adhesives, the property modifying petroleum additives, and plastics.
The pharmaceutical industry began in the last decade of the 19th century when acetylsalicylic acid (aspirin) manufacture was started in Germany by Bayer.
Biochemistry, the chemistry of living organisms, their structure, and interactions in vitro and inside living systems, has only started in the 20th century, opening up a brand new chapter of organic chemistry with enormous scope.
Classification of organic substances[edit]
Description and nomenclature[edit]
Classification is not possible without having a full description of the individual compounds. In contrast with inorganic chemistry, in which describing a chemical compound could be achieved by simply enumerating the chemical symbols of the elements present in the compound together with the number of these elements in the molecule, in organic chemistry the relative arrangement of the atoms within a molecule has to be added for a full description.
One way of describing the molecule is by drawing its structural formula. Because of the complexity this method has changed, becoming simplified over the years. The latest version is the line formula, which achieves simplicity without introducing ambiguity, whilst representing carbon and hydrogen by implication. The disadvantage which arises from the fact that structural formulae cannot be described by words, and that they are not easily printable does not arise when the structure is described by the organic nomenclature.
Because of the difficulty due to the very large number and variety of organic compounds, chemists realized early on that the establishment of an internationally accepted system of naming organic compounds was of paramount importance. The Geneva Nomenclature was born in 1892 as a result of a number of international meetings on the subject.
It was also realized that as the family of organic compounds grew, the system would have to be expanded and modified. This task was ultimately taken on by the International Union on Pure and Applied Chemistry, IUPAC.
Recognizing the fact that in the branch of Biochemistry, the complexity of organic structures increases, the IUPAC organization joined forces with IUBMB, the International Union of Biochemistry and Molecular Biology, to produce a list of joint recommendations on nomenclature.
Further on, as number and complexity grew, new recommendations were made within IUPAC for simplification. The first such recommendation was presented in 1951 when a cyclic benzene structure was named a cyclophane. Later recommendations extended the method to the simplification of other complex cyclic structures, including for instance heterocyclics as well, and named such structures phanes.
For ordinary communication, to spare a tedious description, the official IUPAC naming recommendations are not always followed in practice except when it is necessary to give a concise definition to a compound, or when the IUPAC name is simpler (viz. ethanol against ethyl alcohol). Otherwise the common or trivial name may be used, often derived from the source of the compound.
Classification[edit]
In summary: organic substances are classified by their molecular structural arrangement and by what other atoms are present with the chief (carbon) constituent in their makeup, whilst in a structural formula, hydrogen is implicitly assumed to occupy all free valencies of an appropriate carbon atom, which remain after accounting for branching, other element(s), and/or multiple bonding.
Hydrocarbons and Functional Groups[edit]
Classification normally starts with the hydrocarbons: compounds that contain only carbon and hydrogen. For sub-classes see below. Other elements present themselves in atomic configurations called functional groups, which have a decisive influence on the chemical and physical characteristics of the compound; thus those containing the same atomic formations have similar characteristics, which may be miscibility with water, acidity/ alkalinity, chemical reactivity, oxidation resistance, or others. Some functional groups are also radicals, similar to those in inorganic chemistry and defined as atomic configurations which pass during chemical reactions from one chemical compound into another without change.
Some of the elements of the functional groups (O, S, N, halogens) may stand alone and the group name is not strictly appropriate, but because of their decisive effect on the way they modify the characteristics of the hydrocarbons in which they are present they are classed with the functional groups, and their specific effect on the properties lends excellent means for characterization and classification.
Referring to the hydrocarbon types below, many, if not all of the functional groups which are typically present within aliphatic compounds are also represented within the aromatic and alicyclic group of compounds, unless they are dehydrated, which would lead to non-reacting co-optional groups.
Reference is made here again to the organic nomenclature, which shows an extensive (if not comprehensive) number of classes of compounds according to the presence of various functional groups, based on the IUPAC recommendations, but also some based on trivial names. Putting compounds in sub-classes becomes more difficult when more than one functional group is present.
Two overarching chain type categories exist: Open Chain aliphatic compounds and Closed Chain cyclic compounds. Those in which both open chain and cyclic parts are present are normally classed with the latter.
Aliphatic compounds[edit]
The aliphatic hydrocarbons are subdivided into three groups, homologous series according to their state of saturation: paraffins alkanes without any double or triple bonds, olefins alkenes with double bonds, which can be mono-olefins with a single double bond, di-olefins, or di-enes with two, or poly-olefins with more. The third group with a triple bond is named after the name of the shortest member of the homologue series as the acetylenes alkynes. The rest of the group is classed according to the functional groups present.
From another aspect aliphatics can be straight-chain or branched-chain compounds, and the degree of branching also affects characteristics, like octane number or cetane number in petroleum chemistry.
Aromatic and alicyclic compounds[edit]
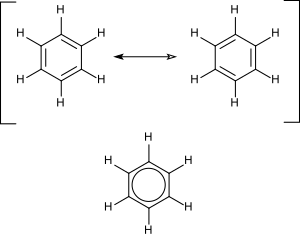
Cyclic compounds can, again, be saturated or unsaturated. Because of the bonding angle of carbon, the most stable configuration of the cyclic compounds contains six carbon atoms, but rings with five carbon atoms are also frequent, and others are rarer. The cyclic hydrocarbons divide into alicyclics and aromatics also called arenes.
Of the alicyclic compounds the cycloalkanes (cycloparaffins) do not contain double bonds, whilst cycloalkenes (cyclo-olefins) do. The simplest member of the cycloalkane family is cyclopropane. A notable group amongst the alicyclics is represented by the terpenes.
What is different in aromatic hydrocarbons is that they contain conjugated or alternating double bonds. One of the simplest examples of this is benzene the structure of which was formulated by Kekulé who first proposed the delocalization or resonance principle for explaining its structure.
The characteristics of the cyclic hydrocarbons are again altered if there are functional groups present, but additionally here some of the elements which are classed with the functional groups can form part of the ring itself. The compounds which only have carbon and hydrogen in their ring(s) are also called homocyclic, those with other elements in the ring heterocyclic and the atom substituting the carbon is a heteroatom.
The heteroatom of heterocyclic molecules is generally O, S, or N, but most often nitrogen, and the heterocyclics of live systems are compounds with nitrogen. Examples of groups among the heterocyclics are the aniline dyes, the great majority of the compounds discussed in biochemistry such as alkaloids, many compounds related to vitamins, steroids, nucleic acids and also numerous medicines. Constructionally simple representatives of the group are pyrrole (5-membered) and indole (6-membered).
Polymers[edit]
One important property of carbon in organic chemistry is that it can form certain compounds, the individual molecules of which are capable of attaching themselves to one another, thereby forming a chain or a network. The process is called polymerization and the chains or networks polymers, whilst the source compound is a monomer. Two main groups of polymers exist: those artificially manufactured are referred to as industrial polymers [3] or synthetic polymers and those naturally occurring as biopolymers.
Since the invention of the first artificial polymer, bakelite, the family has quickly grown with the invention of others. Common synthetic organic polymers are polyethylene or polythene, polypropylene, nylon, teflon or PTFE, polystyrene, polyesters, polymethylmethacrylate (commonly known as perspex or plexiglas) polyvinylchloride or PVC, and polyisobutylene important artificial or synthetic rubber also the polymerised butadiene, a rubber component.
The examples are generic terms, and many varieties of each of these may exist, with their physical characteristics fine-tuned for a specific use. Changing the conditions of polymerization changes the chemical composition of the product by altering chain length, or branching, or the tacticity. With a single monomer as a start, the product is a homopolymer. Further, secondary component(s) may be added to create a heteropolymer (co-polymer) and the degree of clustering of the different components can also be controlled. Physical characteristics, such as hardness, density, mechanical or tensile strength, abrasion resistance, heat resistance, transparency, color, etc. will depend on the final composition.
The only other element that can produce polymers is silicon. The silicones, however, show one major difference from carbon-based polymers, inasmuch as unlike the direct C-C bonds of those based on carbon in silicones the Si atoms are joined indirectly through oxygen links.
Biomolecules[edit]
Biomolecular chemistry is a major category within organic chemistry. Many complex multi-functional group molecules are important in living organisms. Some are long-chain biopolymers. The main classes are carbohydrates, amino acids and proteins, polysaccharides, lipids, and nucleic acids.
Others[edit]
Organic compounds containing bonds of carbon to nitrogen, oxygen, and the halogens are not normally grouped separately. Others are sometimes put into major groups within organic chemistry and discussed under titles such as organosulfur chemistry, organometallic chemistry, organophosphorus chemistry and organosilicon chemistry.
Characteristics of organic substances[edit]
Organic compounds are generally covalently bonded. This allows for unique structures such as long carbon chains and rings. The reason carbon is excellent at forming unique structures and that there are so many carbon compounds is that carbon atoms form very stable covalent bonds with one another (catenation). In contrast to inorganic materials, organic compounds typically melt, boil, sublimate, or decompose below 300°C. Neutral organic compounds tend to be less soluble in water compared to many inorganic salts, with the exception of certain compounds such as ionic organic compounds and low molecular weight alcohols and carboxylic acids where hydrogen bonding occurs.
Organic compounds tend rather to dissolve in organic solvents which are either pure substances like ether or ethyl alcohol, or mixtures, such as the paraffinic solvents such as the various petroleum ethers and white spirits, or the range of pure or mixed aromatic solvents obtained from petroleum or tar fractions by physical separation or by chemical conversion. Solubility in the different solvents depends upon the solvent type and on the functional groups if present. Solutions are studied by the science of Physical Chemistry. Like inorganic salts, organic compounds may also form crystals. A unique property of carbon in organic compounds is that its valency does not always have to be taken up by atoms of other elements, and when it is not, a condition termed unsaturation results. In such cases, we talk about carbon-carbon double bonds or triple bonds. Double bonds alternating with single bonds in a chain are called conjugated double bonds. An aromatic structure is a special case in which the conjugated chain is a closed ring.
Molecular structure elucidation[edit]
Organic compounds consist of carbon atoms, hydrogen atoms, and functional groups. The valence number of carbon is 4, hydrogen is 1, and functional groups are generally 1. From the number of carbon atoms and hydrogen atoms in a molecule, the degree of unsaturation can be obtained. Many, but not all, structures can be envisioned by the simple valence rule that there will be one bond for each valence number. The knowledge of the chemical formula for an organic compound is not sufficient information because many isomers can exist.
Organic compounds often exist as mixtures. Because many organic compounds have relatively low boiling points and/or dissolve easily in organic solvents, there exist many methods for separating mixtures into pure constituents specific to organic chemistry such as distillation, crystallization, and chromatography techniques. There exist several methods for deducing the structure of an organic compound. Methods in general usage are (in alphabetical order):
- Crystallography: This is the most precise method for determining molecular geometry; however, it is very difficult to grow crystals of sufficient size and high quality to get a clear picture, so it remains a secondary form of analysis.
- Elemental analysis: A destructive method used to determine the elemental composition of a molecule.
- Infrared spectroscopy: Chiefly used to determine the presence (or absence) of certain functional groups.
- Mass spectrometry: Used to determine the molecular weight of a compound and from the fragmentation pattern its structure.
- Nuclear magnetic resonance (NMR) spectrometry identifies different nuclei from their chemical environment.
- UV/VIS spectroscopy: Used to determine the degree of conjugation in the system
Additional methods are provided by analytical chemistry.
Organic reactions[edit]
Organic reactions are chemical reactions involving organic compounds. While pure hydrocarbons undergo certain limited classes of reactions, many more reactions which organic compounds undergo are largely determined by functional groups. The general theory of these reactions involves careful analysis of such properties as the electron affinity of key atoms,bond strengths, and steric hindrance. These issues can determine the relative stability of short-lived reactive intermediates, which usually directly determine the path of the reaction. An example of a common reaction is a substitution reaction written as:
- Nu− + C-X → C-Nu + X−
where X is some functional group and Nu is a nucleophile.
There are many important aspects of a specific reaction. Whether it will occur spontaneously or not is determined by the Gibbs free energy change of the reaction. The heat that is either produced or needed by the reaction is found from the total Enthalpy change. Other concerns include whether side reactions occur from the same reaction conditions. Any side reactions which occur typically produce undesired compounds which may be anywhere from very easy or very difficult to separate from the desired compound.
Attribution[edit]
- Some content on this page may previously have appeared on Wikipedia.
References[edit]
- ↑ Robert T. Morrison, Robert N. Boyd, and Robert K. Boyd, Organic Chemistry, 6th edition (Benjamin Cummings, 1992, ISBN 0-13-643669-2) - this is "Morrison and Boyd", a classic textbook
- ↑ Richard F. and Sally J. Daley, Organic Chemistry, www.ochem4free.com, Online organic chemistry textbook.
- ↑ "industrial polymers, chemistry of." Encyclopædia Britannica. 2006
 KSF
KSF