Petroleum crude oil
 From Citizendium - Reading time: 14 min
From Citizendium - Reading time: 14 min

The famous Lucas Gusher oil well of 1901 in Spindletop, Texas.
Petroleum crude oil, or simply crude oil, is a naturally occurring, flammable liquid found primarily in underground geological formations and consists of a complex mixture of hydrocarbons of various molecular weights plus other organic compounds.
The Latin word petroleum was first used to describe petroleum crude oil by the German mineralogist Georg Bauer (also known as Georgius Agricola) in the treatise De Natura Fossilium, published in 1546[1] The Greek word for petroleum is πετρέλαιον, meaning "rock oil".
The first oil well drilled at Spindletop in southeast Texas, known as the "Lucas Gusher", was completed when petroleum crude oil gushed forth on January 10, 1901. The oil reservoir underneath Spindletop was formed by a salt dome (see the "Crude oil sources" section below) and become known as the oil well that started the birth of the oil industry in Texas. It initially produced about 100,000 barrels per day (16,000 cubic metres per day), more than the combined production from all of the oil wells then existing in the United States.
Composition of crude oil[edit]
|
|
Both crude oil and natural gas are predominantly mixtures of hydrocarbons. At typical ambient conditions of pressure and temperature, the lower molecular weight hydrocarbons methane, ethane, propane and butane occur as gases, while the higher molecular weight ones (pentane and higher) are in the form of liquids or solids. However, in the underground oil reservoirs the proportion which is gas or liquid varies depending on the subsurface conditions, and on the phase diagram of the petroleum mixture.[2]
Crude oil consists mostly of hydrocarbons with small amounts of other organic chemical compounds that may contain nitrogen, oxygen or sulphur. It may also contain trace amounts of metals such as iron, nickel, copper and vanadium. The exact elemental composition varies widely from formation to formation but the proportion of chemical elements vary over fairly narrow limits.[3] The distribution of the different types of hydrocarbons in petroleum also varies considerably from one crude oil reservoir to another which means that the properties of the various crude oils are quite different.[2]
The average elemental composition of petroleum crude oil and the average distribution of the different hydrocarbons in the various crude oils are shown in the adjacent tables.
- The hydrocarbons in crude oil
Petroleum is a mixture of a very large number of different hydrocarbons. The most common hydrocarbons found in petroleum crude oil are linear or branched alkanes (also called paraffins), cycloalkanes (also called cyclic paraffins or naphthenes), aromatic hydrocarbons, or much more complicated chemicals like asphaltenes which may have a molecular weight of 800 to 2500.[4][5] .
The alkanes present in crude oil are saturated hydrocarbons, with linear or branched chains, which contain only carbon and hydrogen atoms and have the general formula of CnH2n+2. They generally have from 4 to 40 carbon atoms per molecule, although some molecules may be present that have less than 5 or more than 40 carbon atoms.
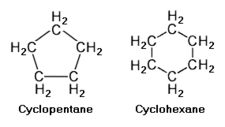
The cycloalkanes are also saturated hydrocarbons, but they have one or more rings of carbon atoms to which hydrogen atoms are attached. The general formula for cycloalkane having a single ring of carbon atoms (with no side chains) is CnH2n. Cycloalkanes have similar properties to alkanes but have higher boiling points.
The upper adjacent diagram depicts the chemical structure of cyclopentane and cyclohexane as some examples of cycloalkanes having a single ring.
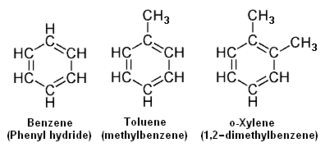
The aromatic hydrocarbons have one or more rings of six carbons, called benzene rings, to which
hydrogen atoms are attached. The general formula of the aromatic hydrocarbons having a single ring (and having no side chains) is CnHn.
The lower adjacent diagram depicts the chemical structures of benzene as an example of an aromatic hydrocarbon having a single ring with no side chains, as well as the structures of toluene and o-Xylene as examples of aromatic hydrocarbons having a single benzene ring with one and with two side chains.
Formation of crude oil[edit]
According to generally accepted theory, petroleum is derived from ancient biomass.[6] The theory was initially based on the isolation of molecules from petroleum that closely resembled known biomolecules.
More specifically, crude oil and natural gas are products of heating of ancient organic materials (i.e. kerogen) over geological time. Formation of petroleum occurs from hydrocarbon pyrolysis, in a variety of mostly endothermic reactions at high temperature and/or pressure. Today's oil formed from the preserved remains of prehistoric zooplankton and algae, which had settled to a sea or lake bottom in large quantities under anoxic conditions. Over geological time, the organic matter mixed with mud, and was buried under heavy layers of sediment resulting in high levels of heat and pressure. This process caused the organic matter to change, first into the waxy material known as kerogen, which is found in various oil shales around the world, and then with more heat into liquid and gaseous hydrocarbons via a process known as catagenesis.
Geologists often refer to the temperature range in which oil forms as an "oil window". Below the oil window minimum temperature oil remains trapped in the form of kerogen, and above the window maximum temperature the oil is converted to natural gas through the process of thermal cracking. Although this temperature range is found at different depths below the surface throughout the world, a typical depth for the oil window is 4 – 6 km. Sometimes, oil which is formed at extreme depths may migrate and become trapped at much shallower depths than where it was formed, as in the case of the Athabasca Oil Sands.
Crude oil sources[edit]
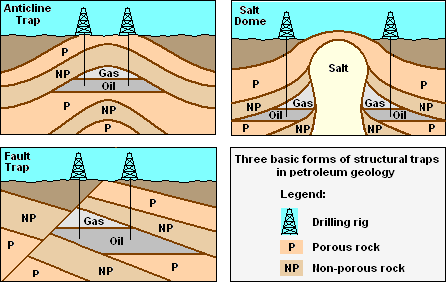
The three basic forms of structural crude oil traps.
- Conventional crude oil reservoirs
Three conditions must be present for oil reservoirs to form, as depicted in the adjacent drawing:
- A reservoir of hydrocarbon material must exist and must have been buried deep enough for subterranean heat and pressure to have transformed it over a long period of time into crude oil.
- A porous, permeable reservoir rock for the crude oil to accumulate in.
- A non-porous, non-permeable cap rock that acts to seal and to prevent the accumulated crude oil from migrating upward and escaping to the surface.
Because most hydrocarbons are lighter than rock or water, they often migrate upward by permeating through porous, permeable rock layers until either reaching the surface or becoming trapped by non-porous, impermeable rocks above. When hydrocarbons are accumulated in a such a trap, an oil reservoir forms from which the oil can be extracted by drilling and pumping as also shown in the adjacent drawing.
- Unconventional oil reservoirs
Oil-eating bacteria biodegrades oil that has escaped to the surface. Oil sands are reservoirs of partially biodegraded oil still in the process of escaping and being biodegraded, but they contain so much migrating oil that, although most of it has escaped, vast amounts are still present—more than can be found in conventional oil reservoirs. The lighter fractions of the crude oil are destroyed first, resulting in reservoirs containing an extremely heavy form of crude oil, called crude bitumen in Canada, or extra-heavy crude oil in Venezuela. These two countries have the world's largest deposits of oil sands.
On the other hand, oil shales are source rocks that have not been exposed to heat or pressure long enough to convert their trapped hydrocarbons into crude oil. Technically speaking, oil shales are not really shales and do not really contain oil, but are usually relatively hard rocks called marls containing a waxy substance called kerogen. The kerogen trapped in the rock can be converted into crude oil using heat and pressure, in a process known as catagenesis, to simulate natural processes. The method has been known for centuries and was patented in 1694 under British Crown Patent No. 330 covering, "A way to extract and make great quantityes of pitch, tarr, and oyle out of a sort of stone." Although oil shales are found in many countries, the United States has the world's largest deposits.[7]
Enhanced oil recovery[edit]
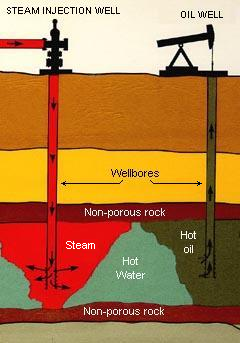
Steam injection for enhanced oil recovery.
The initial or primary phase of recovering oil from from an underground reservoir takes advantage of the natural pressure existing in the the reservoir, assisted by pumps (if needed) to lift the oil to the surface. But only about 10 percent of a reservoir's original-oil-in-place (referred to as OOIP) is typically produced during primary recovery.[8][9][10]
In order to extend the productive life of an oil field, most oil producers have used secondary recovery methods since the late 1940's. Such secondary methods generally involve injecting water into the underground reservoir to displace the oil and drive it into the wellbore where it can be lifted to the surface by pumps. In some cases, natural gas (often produced simultaneously with the oil) is reinjected to maintain reservoir pressure, thus driving the oil into the wellbore. Secondary recovery methods generally raise the overall oil recovery to 20 – 40 percent of the original-oil-in-place. Thus, even after the secondary phase of recovery, about 60 – 80 percent of the oil still remains in the reservoir.[8][9][10]
Enhanced Oil Recovery (abbreviated EOR) is a generic term for tertiary techniques used to further increase the recovery of oil from an oil field. Using EOR, 30 – 60 %, or more of the reservoir’s original oil-in-place can be recovered compared with 20 – 40% using primary and secondary recovery. Three major EOR techniques have been found to be commercially viable:.[8][9][10]
- Using steam injection to raise the temperature of the oil, which reduces the oil's viscosity and improves its ability to flow through the reservoir (see adjacent drawing). In the United States, this technique amounts to over 50 percent of the EOR production.
- Raising the pressure within the reservoir by the injection of a gas such as natural gas, nitrogen or carbon dioxide that expand in the reservoir and push the additional oil to the wellbore. Gas injection accounts for nearly 50 percent of the EOR production in the United States.
- Chemical injection, which involves using long-chained molecules called polymers to increase the effectiveness of water injection, or using detergent-like surfactants to help lower the surface tension that often prevents oil droplets from moving through a reservoir. Chemical techniques account for less than one percent of EOR production in the United States.
Classification of crude oils[edit]
The petroleum industry generally classifies crude oil by the geographic location of the reservoir from which it is produced (e.g. West Texas Intermediate, Brent, or Oman), its API gravity (an oil industry measure of density), and by its sulfur content. Crude oil may be considered light if it has a low density or heavy if it has a high density and it may be referred to as sweet if it contains relatively little sulfur or sour if it contains substantial amounts of sulfur.
Light crude oil is more desirable than heavy oil since it provides a higher yield of gasoline and sweet oil is more desirable than sour oil because it has fewer environmental problems and requires less refining to meet sulfur content standards of refined fuels. Each crude oil has a unique composition and set of physical properties which are delineated by crude oil assays performed in petroleum laboratories.
Some of the common petroleum crude oils (many of which are known as benchmark crude oils) are:
- West Texas Intermediate is a very high-quality, sweet, light oil. It is a North American oil.
- Brent Blend is a blend of 15 oils from the Brent and Ninian oil fields in the East Shetland Basin of the North Sea.
- Dubai Crude is a Middle Eastern, sour oil from Dubai.
- Arabian Crude is a Middle Eastern oil from Saudi Arabia.
- Tapis Crude is a Far Eastern oil from Malaysia.
- Minas Crude is a Far Eastern oil from Indonesia.
- Pembina encompasses crude oils from Western Canada.
- Russian Export Blend is a mixture of several crude oils exported by Russia.
Products produced from crude petroleum[edit]
The petroleum crude oil is refined in petroleum refineries to produce various fuels as well as a number of other products.
- Fuels
- Liquified petroleum gas, commonly referred to as LPG
- Gasoline, also called petrol, in various grades
- Jet fuel in various grades
- Kerosene
- Diesel oil
- Fuel oil
- Other products
- Solvents for various industrial and other uses
- Lubricants such as motor oils and greases
- Petroleum wax
- Sulphur, a byproduct of sulfur removal from fuels.
- Asphalt
- Petroleum coke, used in specialty carbon products or as a solid fuel.
- Petrochemical feed stocks:
- Benzene, toluene and xylenes
- Petroleum naphtha and fuel oils as feedstocks for steam-assisted thermal cracking plants referred to as steam crackers that produce intermediate petrochemical feedstocks
Crude oil statistics[edit]
The three tables below provide the 20008 statistics for the production, consumption and proven reserves of petroleum crude oil of the top nations in each of those categories. The corresponding total world quantities of each category were (See table footnotes for measurement definitions and conversions):
- Production: 73,780,000 bbl/day (11,730,000 m3/day)
- Consumption: approximately 73,780,000 bbl/day (11,730,000 m3/day)
- Proven reserves: 1,332,000,000,000 bbl (211,790,000,000 m3)
As noted in the table footnotes, Canada's proven reserves include the petroleum in their Athabasca Oil Sands. The proven reserves of Venezuela probably include their oil sands also, but that is not explicitly stated in the referenced data sources.
|
|
|
Footnotes for the above tables:
- bbl/d and m3/d are the liquid volume measures of barrels per day and cubic metres per day, respectively. 1 bbl = 0.1590 m3 and 1 m3 = 6.289 bbl
- Proved reserves are those quantities of petroleum crude oil, which, by analysis of geological and engineering data, can be estimated with a high degree of confidence to be commercially recoverable from a given date forward, from known reservoirs and under current economic conditions.
- Canada's crude from oil sands is included in its proven reserves. On that basis, it has the world's second largest oil reserves after Saudi Arabia.
- Canada's production of crude oil from conventional sources is declining, but its production from oil sands is increasing.
- In the terminology of the oil industry, production refers to the quantity of crude oil extracted from reserves, not the literal creation of the product.
History[edit]
- Early history
Petroleum, in one form or another, has been used since ancient times. About 2400 years ago (450 BC), Herodotus describes the oil pits at Ardericca (near Babylon) and the pitch (asphalt) spring of Zacynthus.[13] Natural asphalt deposits in pits were used as mortar for bricks and walls, and for the construction of Babylonian towers as well as for waterproofing boats. Large quantities of asphalt were also found on the banks of the Issus River (a tributary of the Euphrates River).[14] Ancient Persian tablets indicate that petroleum was used for medicinal and lighting purposes by the upper levels of their society.
In about 325 BC, Alexander the Great used flaming torches of petroleum tar or oil to scare his enemies and, in about the first century AD, Plutarch refers to the petroleum found near Ecbatana (Kirkuk) in what is now Iraq. In the fourth century (347 AD), the Chinese are reported to have drilled holes up to 800 feet deep to extract oil by using drill bits attached to bamboo poles.[15][16]
Petroleum ("burning water") was known in Japan during the 7th century, while in Europe the gas springs of the north of Italy led to the adoption in 1226 by the municipality of Salsomaggiore of a salamander surrounded by flames as its emblem.[13]
Marco Polo refers to the oil springs of Baku (in what is now Azerbaijan) towards the end of the 13th century. In 1436, the medicinal properties of the oil found at Tegernsee in the Bavarian Alps gave it the name of "St. Quirinus's Oil". The oil at Pechelbronn in Alsace (now in the northeastern part of France) was discovered in 1498, and the "earthbalsam" of Galicia, an area in East-Central Europe, was known in 1506. In 1595, Sir Walter Raleigh wrote of the lake of pitch (asphalt) in Trinidad.[13] Also in the 1500s, oil from seeps in the Carpathian Mountains of Poland was burned in street lamps of the Polish town of Krosno.[16] In 1594, oil wells were dug by hand at Baku up to 115 feet (135 metres) deep.[15]
1735: Oil sands are mined and the oil extracted at Pechelbronn in Alsace.
1814: One of the first oil wells to produce oil that was marketed was drilled to a depth of 500 feet (150 metres) near Marietta, Ohio. The well was actually drilled for salt water and it just happened to produce about 1 barrel per week of byproduct crude oil which was marketed.
1818: In southeastern Kentucky, another salt water well, known as the Beatty well and located on the banks of the south fork of the Cumberland River, produced upwards of 100 barrels (16 m3) per day. By 1820, oil from this well was being shipped to several other southern states as well as to Europe.
1848: First modern oil well is drilled in Asia, on the Aspheron Peninsula north-east of Baku, by Russian engineer F.N. Semyenov.
1849: Abraham Gesner, a physician and geologist from Nova Scotia, Canada developed a process for distilling kerosene from crude oil. About 7 years later, the kerosene lamp was developed, proving clean burning light.
1854: First oil wells drilled to about 30 to 40 metres (100 to 130 feet) deep in Europe at Bóbrka, near Krosno in Poland by Ignacy Łukasiewicz, a Polish pharmacist. That was followed in 1857 by oil wells drilled in the Carpathian Bend area northeast of Bucharest, Romania.
1858: The first oil well in North America was drilled in Ontario, Canada by James Miller Williams, born in New Jersey and migrated to Canada. That was followed a year later in 1859 by the first oil well drilled in the United States. It was drilled 69 feet (21 metres) deep at Titusville, Pennsylvania by Colonel Edwin Drake and initially produced 25 barrels (4 m3) per day which later decreased to 15 barrels (2 m3)per day.
1859 to 1900: The production of crude oil worldwide increased greatly during this period of time. In the United States, oil production increased from about 2000 barrels (318 m3) in 1859 to about 57,000,000 barrels (9,000,000 m3) in 1900.
Then on January 10, 1901, the Lucas Gusher (see photo at the top of this article) was drilled by Captain Anthony Lucas at Spindletop, Texas and was completed in 1901. Its initial production rate of about 100,000 barrels (16,000 m3) per day was far more than the combined production from all of the oil wells then existing in the United States and heralded the birth of the Texas oil industry.
Today, the petroleum industry is global in its scope. The largest volume products of the industry are fuel oils and gasoline (petrol). Petroleum is also the raw material for many chemical products, including pharmaceuticals, solvents, fertilizers, pesticides, and plastics.
Environmental effects[edit]
The presence of oil has significant social and environmental impacts from accidents and routine activities such as seismic exploration, drilling, and generation of polluting wastes and greenhouse gases.
- Extraction
Oil extraction is costly and sometimes environmentally damaging, although oceanographer Dr. John Hunt of the Woods Hole Oceanographic Institution pointed out in a 1981 paper that over 70% of the reserves in the world are associated with visible macroseepages, and many oil fields are found due to natural seeps. Offshore exploration and extraction of oil disturbs the surrounding marine environment.[17] Extraction may involve dredging, which stirs up the seabed and kills the sea plants that marine creatures need to survive. But at the same time, offshore platforms also form micro-habitats for marine creatures.
Extraction of oil may possibly lead to subsidence at ground level and that can affect ecosystems and waterways as well as sewer and water supply systems. Large oil fields also degrade the aesthetics of rural or wilderness areas.
- Oil spills
Oil spills at sea are generally much more damaging than those on land, since they can spread for hundreds of nautical miles in a thin oil slick which can cover beaches with a thin coating of oil. Such spills can kill sea birds, mammals, shellfish and other organisms it coats. Oil spills on land are more readily containable if a makeshift earthen dam can be rapidly bulldozed around the spill site before most of the oil escapes, and land animals can avoid the oil more easily.
Crude oil and refined fuel spills from tanker ship accidents have damaged natural ecosystems in Alaska, the Galapagos Islands, France and many other places. The quantity of oil spilled from tanker ships during accidents has ranged from a few hundred tons to several hundred thousand tons (e.g., the tankers Atlantic Empress and Amoco Cadiz). Smaller spills have already proven to have a great impact on ecosystems, such as the oil spill from the Exxon Valdez tanker.
Control of oil spills is difficult, requires ad hoc methods, and often a large amount of manpower. The dropping of bombs and incendiary devices from aircraft on the Torrey Canyon tanker wreck produced poor results.[18] Modern techniques would include pumping the oil from the wreck, like in the Prestige tanker oil spill or the oil spill from the Erika tanker.[19]
References[edit]
- ↑ Bauer Georg (1546). De Natura Fossilium. Translated in 1955 by Mark C. Bandy and Jean A. Bandy
- ↑ 2.0 2.1 Norman J. Hyne (2001). Nontechnical Guide to Petroleum Geology, Exploration, Drilling, and Production. PennWell, pages 1-4. ISBN 0-87814-823-X.
- ↑ James G. Speight (1999). The Chemistry and Technology of Petroleum. Marcel Dekker, pages 215-216. ISBN 0-8247-0217-4.
- ↑ Oliver Mullins and Eric Sheu (Editors) (1999). Structure & Dynamics of Asphaltenes, 1st Edition. Springer. ISBN 0-306-45930-2. (See Chapter 1, page 17)
- ↑ Note: There are many other values in the technical literature for the molecular weight of asphaltenes and there does not appear to be a consensus as to which values are more correct.
- ↑ Keith A. Kvenvolden (2006), Organic geochemistry – A retrospective of its first 70 years, Organic Geochemistry, 37 pp. 1–11.
- ↑ Lambertson, Giles. Oil Shale: Ready to Unlock the Rock, Construction Equipment Guide, February 16, 2008.
- ↑ 8.0 8.1 8.2 Enhanced Oil Recovery From the website of the U.S. Department of Energy
- ↑ 9.0 9.1 9.2 Exploration & Production Technologies: Improved Recovery - Enhanced Oil Recovery From the website of the National Energy Technology Laboratory (NETL), U.S. Department of Energy
- ↑ 10.0 10.1 10.2 Enhanced Oil Recovery From the website of the Meteor Oil & Gas Corporation
- ↑ 11.0 11.1 Country Energy Profiles Energy Information Administration, U.S. Department of Energy
- ↑ Proved Reserves of Oil by Country CIA World Factbook
- ↑ 13.0 13.1 13.2 13.3 Petroleum Article originally appearing in Volume V21, Page 321 of the 1911 Encyclopedia Britannica (which is now in the public domain).
- ↑ History of Petroleum Joseph Wells, University of Notre Dame.
- ↑ 15.0 15.1 15.2 The History of the Oil Industry from the website of the San Juaquin Geological Society
- ↑ 16.0 16.1 16.2 History of the World Petroleum Industry (Key Dates)
- ↑ Waste discharges during the offshore oil and gas activity by Stanislave Patin, translated by Elena Cascio.
- ↑ Edward Cowan (1968). Oil and water - the Torrey Canyon disaster. Lippincott.
- ↑ Pumping of the Erika cargo
 KSF
KSF