Superionic water
Topic: Astronomy
 From HandWiki - Reading time: 6 min
From HandWiki - Reading time: 6 min
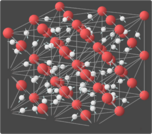
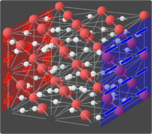
Superionic water, also called superionic ice or ice XVIII,[1] is a phase of water that exists at extremely high temperatures and pressures. In superionic water, water molecules break apart and the oxygen ions crystallize into an evenly spaced lattice while the hydrogen ions float around freely within the oxygen lattice.[2] The freely mobile hydrogen ions make superionic water almost as conductive as typical metals, making it a superionic conductor.[1] It is one of the 19 known crystalline phases of ice. Superionic water is distinct from ionic water, which is a hypothetical liquid state characterized by a disordered soup of hydrogen and oxygen ions.
While theorized for decades, it was not until the 1990s that the first experimental evidence emerged for superionic water. Initial evidence came from optical measurements of laser-heated water in a diamond anvil cell,[3] and from optical measurements of water shocked by extremely powerful lasers.[4] The first definitive evidence for the crystal structure of the oxygen lattice in superionic water came from x-ray measurements on laser-shocked water which were reported in 2019.[1]
If it were present on the surface of the Earth, superionic ice would rapidly decompress. In May 2019, scientists at the Lawrence Livermore National Laboratory (LLNL) were able to synthesize superionic ice, confirming it to be almost four times as dense as normal ice and black in color.[5][4][6]
Superionic water is theorized to be present in the mantles of giant planets such as Uranus and Neptune.[7][8]
Properties
(As of 2013), it is theorized that superionic ice can possess two crystalline structures. At pressures in excess of 50 GPa (7,300,000 psi) it is predicted that superionic ice would take on a body-centered cubic structure. However, at pressures in excess of 100 GPa (15,000,000 psi), and temperatures above 3,140 degrees Fahrenheit, it is predicted that the structure would shift to a more stable face-centered cubic lattice.[9] The ice appears black in color.[4][10]
History of theoretical and experimental evidence
Demontis et al. made the first prediction for superionic water using classical molecular dynamics simulations in 1988.[11] In 1999, Cavazzoni et al. predicted that such a state would exist for ammonia and water in conditions such as those existing on Uranus and Neptune.[12] In 2005 Laurence Fried led a team at Lawrence Livermore National Laboratory to recreate the formative conditions of superionic water. Using a technique involving smashing water molecules between diamonds and super heating it with lasers they observed frequency shifts which indicated that a phase transition had taken place. The team also created computer models which indicated that they had indeed created superionic water.[8] In 2013 Hugh F. Wilson, Michael L. Wong, and Burkhard Militzer at the University of California, Berkeley published a paper predicting the face-centered cubic lattice structure that would emerge at higher pressures.[9]
Additional experimental evidence was found by Marius Millot and colleagues in 2018 by inducing high pressure on water between diamonds and then shocking the water using a laser pulse.[4][13]
2018–2019 experiments
In 2018, researchers at LLNL squeezed water between two pieces of diamond with a pressure of 2,500 MPa (360,000 psi). The water was squeezed into type VII ice, which is 60 percent denser than normal water.[14]
The compressed ice was then transported to the University of Rochester where it was blasted by a pulse of laser light. The reaction created conditions like those inside of ice giants such as Uranus and Neptune by heating up the ice thousands of degrees under a pressure a million times greater than the Earth's atmosphere in only 10 to 20 billionths of a second. The experiment concluded that the current in the conductive water was indeed carried by ions rather than electrons and thus pointed to the water being superionic.[14] More recent experiments from the same Lawrence Livermore National Laboratory team used x-ray crystallography on laser-shocked water droplets to determine that the oxygen ions enter a face-centered-cubic phase, which was dubbed ice XVIII and reported in the journal Nature in May 2019.[1]
Existence in ice giants
It is theorized that the ice giant planets Uranus and Neptune hold a layer of superionic water.[15] Machine learning and free-energy methods predict close-packed superionic phases to be stable over a wide temperature and pressure range, and a body-centred cubic superionic phase to be kinetically favoured, but stable over a small window of paramaters.[16]
On the other hand, there are also studies that suggest that other elements present inside the interiors of these planets, particularly carbon, may prevent the formation of superionic water.[17][18]
Applications
Water is widely known for its existence in three different phases i.e solid, liquid and gas just like any other substance or component that exists. However, Wang. et al have discovered a high-pressure ionic phase of water ice. This ice is known to change its structural configuration according to high and low temperatures. Some of the biggest problems industries are addressed using the existence of these three standard states of water but often operations are carried out at very high and low pressures and temperatures and knowledge of this physical/structural property of water ice might help us understand the material and energy exchange in a more detailed and accurate way. It could be understood whether how this change in configuration affects the outcome of the operation and by how much. It is highly likely that this may provide a deeper understanding of the supercritical water that is used in the industries. A well-developed understanding of this phenomenon might help us design processes/operations in a more efficient way by avoiding many losses.[18]
References
- ↑ 1.0 1.1 1.2 1.3 Millot, Marius; Coppari, Federica; Rygg, J. Ryan; Correa Barrios, Antonio; Hamel, Sebastien; Swift, Damian C.; Eggert, Jon H. (8 May 2019). "Nanosecond X-ray diffraction of shock-compressed superionic water ice". Nature 569 (7755): 251–255. doi:10.1038/s41586-019-1114-6. PMID 31068720. https://www.osti.gov/biblio/1568026.
- ↑ Weird water lurking inside giant planets, New Scientist,01 September 2010, Magazine issue 2776.
- ↑ Goncharov, Alexander F. et al. (2005). "Dynamic Ionization of Water under Extreme Conditions". Phys. Rev. Lett. 94 (12): 125508. doi:10.1103/PhysRevLett.94.125508. PMID 15903935. https://digital.library.unt.edu/ark:/67531/metadc1417703/m2/1/high_res_d/15015926.pdf.
- ↑ 4.0 4.1 4.2 4.3 Millot, Marius (5 February 2018). "Experimental evidence for superionic water ice using shock compression". Nature Physics 14 (3): 297–302. doi:10.1038/s41567-017-0017-4. Bibcode: 2018NatPh..14..297M. https://www.osti.gov/biblio/1542614.
- ↑ Valich, Lindsey. "'Exotic' form of ice both solid and liquid". University of Rochester. https://www.rochester.edu/newscenter/superionic-ice-solid-liquid-381972/.
- ↑ Sokol, Joshua (2019-05-12). "A Bizarre Form of Water May Exist All Over the Universe". Wired. ISSN 1059-1028. https://www.wired.com/story/a-bizarre-form-of-water-may-exist-all-over-the-universe/. Retrieved 2019-05-13.
- ↑ Chang, Kenneth (5 February 2018). "Newly Discovered Form of Water Ice Is 'Really Strange' – Long theorized to be found in the mantles of Uranus and Neptune, the confirmation of the existence of superionic ice could lead to the development of new materials.". The New York Times. https://www.nytimes.com/2018/02/05/science/superionic-water-neptune-uranus.html. Retrieved 5 February 2018.
- ↑ 8.0 8.1 Marris, Emma (22 March 2005). "Giant planets may host superionic water". Nature. doi:10.1038/news050321-4.
- ↑ 9.0 9.1 Phys.org, "New phase of water could dominate the interiors of Uranus and Neptune", Lisa Zyga, 25 April 2013
- ↑ Sokol, Joshua (2019-05-12). "A Bizarre Form of Water May Exist All Over the Universe". Wired. ISSN 1059-1028. https://www.wired.com/story/a-bizarre-form-of-water-may-exist-all-over-the-universe/. Retrieved 2019-05-13.
- ↑ Demontis, P. et al. (1988). "New high-pressure phases of ice". Phys. Rev. Lett. 60 (22): 2284–2287. doi:10.1103/PhysRevLett.60.2284. PMID 10038311. http://eprints.uniss.it/377/1/Demontis_P_Articolo_1988_New.pdf.
- ↑ Cavazzoni, C. et al. (1999). "Superionic and Metallic States of Water and Ammonia at Giant Planet Conditions". Science 283 (5398): 44–46. doi:10.1126/science.283.5398.44. PMID 9872734. Bibcode: 1999Sci...283...44C.
- ↑ Sokol, Joshua (2019-05-12). "A Bizarre Form of Water May Exist All Over the Universe". Wired. ISSN 1059-1028. https://www.wired.com/story/a-bizarre-form-of-water-may-exist-all-over-the-universe/. Retrieved 2019-05-13.
- ↑ 14.0 14.1 Chang, Kenneth (2018-02-05). "New Form of Water, Both Liquid and Solid, Is 'Really Strange'" (in en-US). The New York Times. ISSN 0362-4331. https://www.nytimes.com/2018/02/05/science/superionic-water-neptune-uranus.html.
- ↑ Charlie Osolin. "Public Affairs Office: Recreating the Bizarre State of Water Found on Giant Planets". Llnl.gov. https://www.llnl.gov/news/newsreleases/2005/SF-05-04-01.html. Retrieved 24 December 2010.
- ↑ Cheng, Bingqing; Bethkenhagen, Mandy; Pickard, Chris J.; Hamel, Sebastien (2021). "Phase behaviours of superionic water at planetary conditions". Nature Physics 17 (11): 1228–1232. doi:10.1038/s41567-021-01334-9.
- ↑ Chau, Ricky; Hamel, Sebastien; Nellis, William J. (2011). "Chemical processes in the deep interior of Uranus". Nat. Commun. 2: Article number: 203. doi:10.1038/ncomms1198. PMID 21343921.
- ↑ 18.0 18.1 Wang, Yanchao (29 November 2011). "High pressure partially ionic phase of water ice". Nature Communications 2: 563. doi:10.1038/ncomms1566. PMID 22127059.
 |
 KSF
KSF