-
Tetramer of aldehyde dehydrogenase 2 with a space filling model of NAD+ in each active site.[1]
-
The active site of a human mitochondrial aldehyde dehydrogenase 2. Cys302 and Glu268 interact with the aldehyde substrate. The NAD+ is held in place by multiple residues (shown as wires or sticks).[1]
-
The active site of the K487E mutant aldehyde dehydrogenase 2 with a space-filling model of NAD+ in the active site. The amino acid Glu349 is highlighted.[1]
Aldehyde dehydrogenase
Topic: Biology
 From HandWiki - Reading time: 9 min
From HandWiki - Reading time: 9 min
| Aldehyde dehydrogenase (NAD+) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
 Monomer of human aldehyde dehydrogenase 2 (ALDH2) with a space-filling model of NAD+ in the active site.[1] | |||||||||
| Identifiers | |||||||||
| EC number | 1.2.1.3 | ||||||||
| CAS number | 9028-86-8 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| Gene Ontology | AmiGO / QuickGO | ||||||||
| |||||||||
Aldehyde dehydrogenases (EC 1.2.1.3) are a group of enzymes that catalyse the oxidation of aldehydes.[2] They convert aldehydes (R–C(=O)–H) to carboxylic acids (R–C(=O)–O–H). The oxygen comes from a water molecule. To date, nineteen ALDH genes have been identified within the human genome. These genes participate in a wide variety of biological processes including the detoxification of exogenously and endogenously generated aldehydes.
Function
Aldehyde dehydrogenase is a polymorphic enzyme[3] responsible for the oxidation of aldehydes to carboxylic acids.[3] There are three different classes of these enzymes in mammals: class 1 (low Km, cytosolic), class 2 (low Km, mitochondrial), and class 3 (high Km, such as those expressed in tumors, stomach, and cornea). In all three classes, constitutive and inducible forms exist. ALDH1 and ALDH2 are the most important enzymes for aldehyde oxidation, and both are tetrameric enzymes composed of 54 kDa subunits. These enzymes are found in many tissues of the body but are at the highest concentration in the liver.[3]
Active site
The active site of the aldehyde dehydrogenase enzyme is largely conserved throughout the different classes of the enzyme and, although the number of amino acids present in a subunit can change, the overall function of the site changes little. The active site binds to one molecule of an aldehyde and one molecule of either NAD+ or NADP+, which functions as a cofactor. Cysteine and glutamate molecules interact with the aldehyde substrate. Many other residues will interact with NAD(P)+ to hold it in place. Magnesium may be used to help the enzyme function, although the degree to which magnesium assists the enzyme varies between different classes of aldehydes.
Mechanism
The overall reaction catalysed by the aldehyde dehydrogenases is:
In this NAD(P)+-dependent reaction, the aldehyde enters the active site through a channel extending from the surface of the enzyme. The active site contains a Rossmann fold, and interactions between the cofactor and the fold allow for the action of the active site.[4]
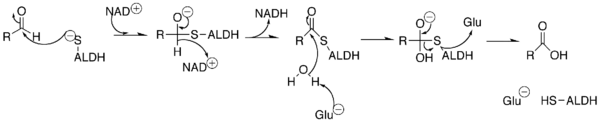 |
A sulfur from a cysteine in the active site makes a nucleophilic attack on the carbonyl carbon of the aldehyde. The hydrogen is kicked off as a hydride and attacks NAD(P)+ to make NAD(P)H. The enzyme's active site then goes through an isomorphic change whereby the NAD(P)H is moved, creating room for a water molecule to access the substrate. The water is primed by a glutamate in the active site, and the water makes a nucleophilic attack on the carbonyl carbon, kicking off the sulfur as a leaving group.
Researchers at the University of Tsukuba found that durian extract inhibited aldehyde dehydrogenase activity, lending credence to an Asian folklore warning against consuming durian with alcohol.[5]
Pathology (aldehyde dehydrogenase deficiency)
Template:Alcohol metabolism formulaeALDH2 plays a crucial role in maintaining low blood levels of acetaldehyde during alcohol oxidation.[7] In this pathway (ethanol to acetaldehyde to acetate), the intermediate structures can be toxic, and health problems arise when those intermediates cannot be cleared.[3] When high levels of acetaldehyde occur in the blood, facial flushing, lightheadedness, palpitations, nausea, and general “hangover” symptoms occur. These symptoms are indicative of a medical condition known as the alcohol flush reaction, also known as “Asian flush” or “Oriental flushing syndrome”.[8]
There is a mutant form of aldehyde dehydrogenase, termed ALDH2*2, wherein a lysine residue replaces a glutamate in the active site at position 487 of ALDH2.[9] Homozygous individuals with the mutant allele have almost no ALDH2 activity, and those heterozygous for the mutation have reduced activity. Thus, the mutation is partially dominant.[3] The ineffective homozygous allele works at a rate of about 8% of the normal allele, for it shows a higher Km for NAD+ and has a higher maximum velocity than the wild-type allele.[3] This mutation is common in Japan, where 41% of a non-alcoholic control group were ALDH2 deficient, where only 2–5% of an alcoholic group were ALDH2-deficient. In Taiwan, the numbers are similar, with 30% of the control group showing the deficiency and 6% of alcoholics displaying it.[3] The deficiency is manifested by slow acetaldehyde removal, with low alcohol tolerance perhaps leading to a lower frequency of alcoholism.[3][8]
These symptoms are the same as those observed in people who drink while being treated by the drug disulfiram, which is why disulfiram is used to treat alcoholism. The patients show higher blood levels of acetaldehyde, and become violently ill upon consumption of even small amounts of alcohol.[3] Several drugs (e.g., metronidazole) cause a similar reaction known as disulfiram-like reaction.
Yokoyama et al. found that decreased enzyme activity of aldehyde dehydrogenase-2, caused by the mutated ALDH2 allele, contributes to a higher chance of esophageal and oropharyngolaryngeal cancers. The metabolized acetaldehyde in the blood, which is six times higher than in individuals without the mutation, has shown to be a carcinogen in lab animals. ALDH2*2 is associated with increased odds of oropharyngolaryngeal, esophageal, gastric, colon, and lung cancer. However, they found no connection between increased levels of ALDH2*2 in the blood and an increased risk of liver cancer.[10]
High expression of the genes that encode ALDH1A1 and ALDH2 is associated with a poor prognosis in patients with acute myeloid leukemia.[11]
Demir et al. found that ALDH1 is a potentially important, poor prognostic factor in breast cancer, associated with high histological grade, estrogen/progesteron receptor negativity and HER2 positivity.[12]
Some case-control studies claimed that carriage of ALDH2*2 allele was a risk of late-onset Alzheimer's disease independent of the apolipoprotein E gene (the odds for LOAD in carriers of ALDH2*2 allele almost twice that of non-carriers).[13] Moreover, ALDH gene, protein expression and activity are substantially decreased in the substantia nigra of Parkinson's disease patients.[14] These reports are in line with findings implementing toxic lipid oxidation-derived aldehydes in these diseases and in neurodegeneration in general.[15]
Fitzmaurice et al. explored aldehyde dehydrogenase inhibition as a pathogenic mechanism in Parkinson disease. "This ALDH model for PD etiology may help explain the selective vulnerability of dopaminergic neurons in PD and provide a potential mechanism through which environmental toxicants contribute to PD pathogenesis."[16]
Knockout mouse models further confirm the involvement of ALDH family in neurodegeneration. Mice null for ALDH1a1 and ALDH2 exhibit Parkinson's disease-like age-dependent deficits in motor performance and significant increase in biogenic aldehydes.[17]
The ALDH2-/- mice display age-related memory deficits in various tasks, as well as endothelial dysfunction, brain atrophy, and other Alzheimer's disease-associated pathologies, including marked increases in lipid peroxidation products, amyloid-beta, p-tau and activated caspases. These behavioral and biochemical Alzheimer's disease-like deficits were efficiently ameliorated when the ALDH2-/- mice were treated with isotope-reinforced, deuterated polyunsaturated fatty acids (D-PUFA).[18]
Genes
- ALDH1A1, ALDH1A2, ALDH1A3, ALDH1B1, ALDH1L1, ALDH1L2
- ALDH2
- ALDH3A1, ALDH3A2, ALDH3B1, ALDH3B2
- ALDH4A1, ALDH5A1, ALDH6A1, ALDH7A1, ALDH8A1, ALDH9A1, ALDH16A1, ALDH18A1
See also
References
- ↑ 1.0 1.1 1.2 1.3 PDB: 1o02; "Coenzyme isomerization is integral to catalysis in aldehyde dehydrogenase". Biochemistry 42 (23): 7100–9. June 2003. doi:10.1021/bi034182w. PMID 12795606.
- ↑ "Non-P450 aldehyde oxidizing enzymes: the aldehyde dehydrogenase superfamily". Expert Opinion on Drug Metabolism & Toxicology 4 (6): 697–720. June 2008. doi:10.1517/17425255.4.6.697. PMID 18611112.
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 3.6 3.7 3.8 "Overview of the role of alcohol dehydrogenase and aldehyde dehydrogenase and their variants in the genesis of alcohol-related pathology". The Proceedings of the Nutrition Society 63 (1): 49–63. February 2004. doi:10.1079/PNS2003327. PMID 15099407.
- ↑ "The first structure of an aldehyde dehydrogenase reveals novel interactions between NAD and the Rossmann fold". Nature Structural Biology 4 (4): 317–26. April 1997. doi:10.1038/nsb0497-317. PMID 9095201.
- ↑ "Durians and booze: Worse than a stinking hangover". http://newscientist.com/article/mg20327253-200-durians-and-booze-worse-than-a-stinking-hangover.
- ↑ Figure 11-4 in: Rod Flower; Humphrey P. Rang; Maureen M. Dale; Ritter, James M. (2007). Rang & Dale's pharmacology. Edinburgh: Churchill Livingstone. ISBN 978-0-443-06911-6. https://archive.org/details/rangdalespharmac0006dale.
- ↑ Edenberg, Howard J.; McClintick, Jeanette N. (2018). "Alcohol Dehydrogenases, Aldehyde Dehydrogenases, and Alcohol Use Disorders: A Critical Review". Alcoholism: Clinical and Experimental Research 42 (12): 2281–2297. doi:10.1111/acer.13904. ISSN 1530-0277. PMID 30320893.
- ↑ 8.0 8.1 "Alcohol and aldehyde dehydrogenase genotypes and alcoholism in Chinese men". American Journal of Human Genetics 48 (4): 677–81. April 1991. PMID 2014795.
- ↑ "Structure of mitochondrial aldehyde dehydrogenase: the genetic component of ethanol aversion". Structure 5 (5): 701–11. May 1997. doi:10.1016/S0969-2126(97)00224-4. PMID 9195888.
- ↑ "Alcohol-related cancers and aldehyde dehydrogenase-2 in Japanese alcoholics". Carcinogenesis 19 (8): 1383–7. August 1998. doi:10.1093/carcin/19.8.1383. PMID 9744533.
- ↑ Dancik, Garrett; Varisli, Lokman; Tolan, Veysel; Vlahopoulos, Spiros (2023). "Aldehyde Dehydrogenase Genes as Prospective Actionable Targets in Acute Myeloid Leukemia" (in en). Genes (Basel) 14 (9): 1807. doi:10.3390/genes14091807. PMID 37761947.
- ↑ Demir, Hale; Dulgar, Ozgecan; Gulle, Bugra Taygun; Turna, Hande; Ilvan, Sennur (2018-11-07). "Prognostic value of aldehyde dehydrogenase 1 (ALDH1) in invasive breast carcinomas" (in en). Bosnian Journal of Basic Medical Sciences 18 (4): 313–319. doi:10.17305/bjbms.2018.3094. ISSN 1840-4812. PMID 29924962.
- ↑ "Deficiency in mitochondrial aldehyde dehydrogenase increases the risk for late-onset Alzheimer's disease in the Japanese population". Biochemical and Biophysical Research Communications 273 (1): 192–6. June 2000. doi:10.1006/bbrc.2000.2923. PMID 10873585.
- ↑ "Aldehyde dehydrogenase (ALDH) in Alzheimer's and Parkinson's disease". Journal of Neural Transmission 123 (2): 83–90. February 2016. doi:10.1007/s00702-014-1320-1. PMID 25298080.
- ↑ "Neurodegeneration and aldehyde load: from concept to therapeutics". Journal of Psychiatry & Neuroscience 31 (5): 296–7. September 2006. PMID 16951732.
- ↑ "Aldehyde dehydrogenase inhibition as a pathogenic mechanism in Parkinson disease". Proceedings of the National Academy of Sciences of the United States of America 110 (2): 636–41. January 2013. doi:10.1073/pnas.1220399110. PMID 23267077. Bibcode: 2013PNAS..110..636F.
- ↑ "Neurodegeneration and motor dysfunction in mice lacking cytosolic and mitochondrial aldehyde dehydrogenases: implications for Parkinson's disease". PLOS ONE 7 (2): e31522. 2012. doi:10.1371/journal.pone.0031522. PMID 22384032. Bibcode: 2012PLoSO...731522W.
- ↑ "Deuterium-reinforced polyunsaturated fatty acids improve cognition in a mouse model of sporadic Alzheimer's disease". The FEBS Journal 284 (23): 4083–4095. December 2017. doi:10.1111/febs.14291. PMID 29024570.
External links
- Aldehyde+dehydrogenase at the US National Library of Medicine Medical Subject Headings (MeSH)
 |
 KSF
KSF![Tetramer of aldehyde dehydrogenase 2 with a space filling model of NAD+ in each active site.[1]](https://handwiki.org/wiki/images/thumb/d/d6/Tetramer_with_NAD_surface.png/215px-Tetramer_with_NAD_surface.png)
![The active site of a human mitochondrial aldehyde dehydrogenase 2. Cys302 and Glu268 interact with the aldehyde substrate. The NAD+ is held in place by multiple residues (shown as wires or sticks).[1]](https://handwiki.org/wiki/images/thumb/d/d1/Active_Site_with_labeled_amino_acids_and_NAD.png/260px-Active_Site_with_labeled_amino_acids_and_NAD.png)
![The active site of the K487E mutant aldehyde dehydrogenase 2 with a space-filling model of NAD+ in the active site. The amino acid Glu349 is highlighted.[1]](https://handwiki.org/wiki/images/thumb/8/84/Mutant_active_site_of_ALDH2%2A2%2C_labeled.png/252px-Mutant_active_site_of_ALDH2%2A2%2C_labeled.png)
