Anthozoa
Topic: Biology
 From HandWiki - Reading time: 18 min
From HandWiki - Reading time: 18 min
| Anthozoa | |
|---|---|

| |
| Coral outcrop on the Great Barrier Reef | |

| |
| Gorgonian with polyps expanded | |
| Scientific classification | |
| Script error: No such module "Taxobox ranks".: | Animalia |
| Script error: No such module "Taxobox ranks".: | Cnidaria |
| Script error: No such module "Taxobox ranks".: | Anthozoa Ehrenberg, 1834 |
| Subclasses | |
Anthozoa is a class of marine invertebrates which includes the sea anemones, stony corals and soft corals. Adult anthozoans are almost all attached to the seabed, while their larvae can disperse as part of the plankton. The basic unit of the adult is the polyp; this consists of a cylindrical column topped by a disc with a central mouth surrounded by tentacles. Sea anemones are mostly solitary, but the majority of corals are colonial, being formed by the budding of new polyps from an original, founding individual. Colonies are strengthened by calcium carbonate and other materials and take various massive, plate-like, bushy or leafy forms.
Anthozoa is included within the phylum Cnidaria, which also includes the jellyfish, box jellies and parasitic Myxozoa and Polypodiozoa. The two main subclasses of Anthozoa are the Hexacorallia, members of which have six-fold symmetry and includes the stony corals, sea anemones, tube anemones and zoanthids; and the Octocorallia, which have eight-fold symmetry and includes the soft corals and gorgonians (sea pens, sea fans and sea whips), and sea pansies. The smaller subclass, Ceriantharia, consists of the tube-dwelling anemones. Some additional species are also included as incertae sedis until their exact taxonomic position can be ascertained.
Anthozoans are carnivores, catching prey with their tentacles. Many species supplement their energy needs by making use of photosynthetic single-celled algae that live within their tissues. These species live in shallow water and many are reef-builders. Other species lack the zooxanthellae and, having no need for well-lit areas, typically live in deep-water locations.
Unlike other members of this phylum, anthozoans do not have a medusa stage in their development. Instead, they release sperm and eggs into the water. After fertilisation, the planula larvae form part of the plankton. When fully developed, the larvae settle on the seabed and attach to the substrate, undergoing metamorphosis into polyps. Some anthozoans can also reproduce asexually through budding or by breaking in pieces.
Diversity

The name "Anthozoa" comes from the Greek words άνθος (ánthos; "flower") and ζώα (zóa; "animals"), hence ανθόζωα (anthozoa) = "flower animals", a reference to the floral appearance of their perennial polyp stage.[1]
Anthozoans are exclusively marine, and include sea anemones, stony corals, soft corals, sea pens, sea fans and sea pansies. Anthozoa is the largest taxon of cnidarians; over six thousand solitary and colonial species have been described. They range in size from small individuals less than half a centimetre across to large colonies a metre or more in diameter. They include species with a wide range of colours and forms that build and enhance reef systems.[2][3] Although reefs and shallow water environments exhibit a great array of species, there are in fact more species of coral living in deep water than in shallow, and many taxa have shifted during their evolutionary history from shallow to deep water and vice versa.[4]
Phylogeny
Anthozoa is subdivided into three subclasses: Octocorallia, Hexacorallia and Ceriantharia, which form monophyletic groups and generally show differentiating reflections on symmetry of polyp structure for each subclass. The relationships within the subclasses are unresolved.[5]
| Anthozoa |
| |||||||||||||||||||||||||||||||||||||||
Historically, the "Ceriantipatharia" was thought to be a separate subclass but, of the two orders it comprised, Antipatharia is now considered part of Hexacorallia and Ceriantharia is now considered an independent subclass. The extant orders are shown to the right.[5][6]
Hexacorallia includes coral reef builders: the stony corals (Scleractinia), sea anemones (Actiniaria), and zoanthids (Zoantharia). Genetic studies of ribosomal DNA has shown Ceriantharia to be a monophyletic group and the oldest, or basal, order among them.[7]
Classification according to the World Register of Marine Species:[8]
- subclass Hexacorallia
- order Actiniaria — sea anemones
- order Antipatharia — black coral
- order Corallimorpharia — corallimorphs
- order Rugosa †
- order Scleractinia — stony corals
- order Zoantharia — zoanthids
- subclass Octocorallia
- order Alcyonacea — soft corals and gorgonians
- order Helioporacea — blue corals
- order Pennatulacea — pennatules, sea feathers, sea pens, sea pansies
- subclass Ceriantharia — ceriantharians, tube-dwelling anemones
- order Penicillaria
- order Spirularia
- Anthozoa incertae sedis
- genus Aiptasiodes
- order Auloporida †
- genus Sarcinula †
Octocorallia comprises the sea pens (Pennatulacea), soft corals (Alcyonacea), and blue coral (Helioporacea). Sea whips and sea fans, known as gorgonians, are part of Alcyonacea and historically were divided into separate orders.[6]
Ceriantharia comprises the related tube-dwelling anemones. Tube-dwelling anemones or cerianthids look very similar to sea anemones, but belong to an entirely different subclass of anthozoans. They are solitary, living buried in soft sediments. Tube anemones live and can withdraw into tubes, which are made of a fibrous material, which is made from secreted mucus and threads of nematocyst-like organelles, known as ptychocysts.[2]
| Major anthozoan taxa | |||||
|---|---|---|---|---|---|
| Subclass | Order | Image | Example | Characteristics | Distribution |
| Hexacorallia | Actiniaria Sea anemones |
 |
Actinostola sp. | Mostly large, solitary polyps anchored to hard substrates. Often colourful. Zooxanthellate or azooxanthellate.[2] | Worldwide in shallow and deep water, with greatest diversity in tropics.[2] |
| Hexacorallia | Antipatharia Black coral |
 |
Plumapathes pennacea | Bushy colonies with slender branches. Axial skeleton of dark-coloured thorny branches strengthened by a unique, non-collagen protein. Azooxanthellate.[2] | On vertical rock faces of reefs, or in deep water.[2] |
| Hexacorallia | Corallimorpharia Corallimorphs or coral anemones |
 |
Discosoma sp. | Large, solitary polyps similar to sea anemones, but with stumpy columns and large oral discs with many short tentacles. Catch large prey and some species zooxanthellate.[2] | On coral reefs, mostly tropical.[2] |
| Hexacorallia | Rugosa Extinct |
 |
Stereolasma rectum | Extinct order abundant in Middle Ordovician to Late Permian. Solitary or colonial, with a skeleton formed of calcite. Septa develop in multiples of four.[9] | Widespread. |
| Hexacorallia | Scleractinia Stony corals or hard corals |
  |
Fungia fungites Tubastraea coccinea |
Solitary or colonial corals in a vast assortment of sizes and shapes, the stony skeleton being composed of aragonite. Septa develop in multiples of six.[9] Zooxanthellate or azooxanthellate.[2] | Shallow and deep water habitats worldwide, the greatest diversity being in tropical seas.[2] |
| Hexacorallia | Zoantharia Zoanthids |
 |
"Dragon eye" coral Zoanthus sp. |
Small, mostly colonial species joined by coensarc or stolons. No hard skeleton but some incorporate solid matter into fleshy periderm.[2] | Mostly tropical, reef-dwelling species.[2] |
| Octocorallia | Alcyonacea Soft corals and gorgonians |
 185px 185px |
Alcyonium digitatum Mushroom corals |
Colonial and diverse, with polyps almost completely embedded in thick fleshy coenosarc. Gorgonians have a horny skeleton. Zooxanthellate or azooxanthellate.[2] | Worldwide, mostly in tropical and subtropical waters, associated with coral reefs and in deep sea.[2] |
| Octocorallia | Helioporacea Blue corals |
 |
Heliopora coerulea | Octocorals with a massive skeleton composed of aragonite secreted by underside of coenosarc. Zooxanthellate.[10] | Heliopora coerulea is IndoPacific; other species are from the Atlantic and Madagascar.[11] |
| Octocorallia | Pennatulacea Sea pens, sea feathers and sea pansies |
 |
Ptilosarcus gurneyi | Colonial species taking pinnate, radial or club-like forms. Main axis is a single enlarged and elongated polyp. Has several types of specialist polyp. Azooxanthellate.[10] | Worldwide, from lower tidal to 6,000 m (20,000 ft)[12] |
| Ceriantharia | Penicillaria Tube-dwelling anemones |
 |
Arachnanthus sarsi | Solitary individuals with two rings of tentacles living in fibrous tubes in soft sediment. Distinguished from Spirularia by anatomy and cnidom.[13] | In soft sediment, worldwide.[14] |
| Ceriantharia | Spirularia Tube-dwelling anemones |
 |
Cerianthus filiformis | Solitary individuals with two rings of tentacles living in fibrous tubes. Distinguished from Penicillaria by anatomy and cnidom.[13] | In soft sediment, worldwide.[14] |
Anatomy
The basic body form of an anthozoan is the polyp. This consists of a tubular column topped by a flattened area, the oral disc, with a central mouth; a whorl of tentacles surrounds the mouth. In solitary individuals, the base of the polyp is the foot or pedal disc, which adheres to the substrate, while in colonial polyps, the base links to other polyps in the colony.[2]
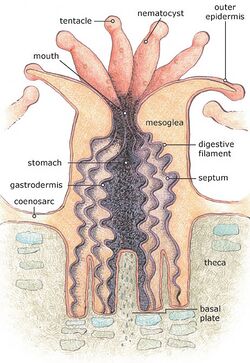
The mouth leads into a tubular pharynx which descends for some distance into the body before opening into the coelenteron, otherwise known as the gastrovascular cavity, that occupies the interior of the body. Internal tensions pull the mouth into a slit-shape, and the ends of the slit lead into two grooves in the pharynx wall called siphonoglyphs. The coelenteron is subdivided by a number of vertical partitions, known as mesenteries or septa. Some of these extend from the body wall as far as the pharynx and are known as "complete septa" while others do not extend so far and are "incomplete". The septa also attach to the oral and pedal discs.[2]
The body wall consists of an epidermal layer, a jellylike mesogloea layer and an inner gastrodermis; the septa are infoldings of the body wall and consist of a layer of mesogloea sandwiched between two layers of gastrodermis. In some taxa, sphincter muscles in the mesogloea close over the oral disc and act to keep the polyp fully retracted. The tentacles contain extensions of the coelenteron and have sheets of longitudinal muscles in their walls. The oral disc has radial muscles in the epidermis, but most of the muscles in the column are gastrodermal, and include strong retractor muscles beside the septa. The number and arrangement of the septa, as well as the arrangement of these retractor muscles, are important in anthozoan classification.[2]
The tentacles are armed with nematocysts, venom-containing cells which can be fired harpoon-fashion to snare and subdue prey. These need to be replaced after firing, a process that takes about forty-eight hours. Some sea anemones have a circle of acrorhagi outside the tentacles; these long projections are armed with nematocysts and act as weapons. Another form of weapon is the similarly armed acontia (threadlike defensive organs) which can be extruded through apertures in the column wall. Some stony corals employ nematocyst-laden "sweeper tentacles" as a defence against the intrusion of other individuals.[2]
Many anthozoans are colonial and consist of multiple polyps with a common origin joined by living material. The simplest arrangement is where a stolon runs along the substrate in a two dimensional lattice with polyps budding off at intervals. Alternatively, polyps may bud off from a sheet of living tissue, the coenosarc, which joins the polyps and anchors the colony to the substrate. The coenosarc may consist of a thin membrane from which the polyps project, as in most stony corals, or a thick fleshy mass in which the polyps are immersed apart from their oral discs, as in the soft corals.[2]
The skeleton of a stony coral in the order Scleractinia is secreted by the epidermis of the lower part of the polyp; this forms a corallite, a cup-shaped hollow made of calcium carbonate, in which the polyp sits. In colonial corals, following growth of the polyp by budding, new corallites are formed, with the surface of the skeleton being covered by a layer of coenosarc. These colonies adopt a range of massive, branching, leaf-like and encrusting forms.[15] Soft corals in the subclass Octocorallia are also colonial and have a skeleton formed of mesogloeal tissue, often reinforced with calcareous spicules or horny material, and some have rod-like supports internally.[16] Other anthozoans, such as sea anemones, are naked; these rely on a hydrostatic skeleton for support. Some of these species have a sticky epidermis to which sand grains and shell fragments adhere, and zoanthids incorporate these substances into their mesogloea.[2]
Biology

Most anthozoans are opportunistic predators, catching prey which drifts within reach of their tentacles. The prey is secured with the help of sticky mucus, spirocysts (non-venomous harpoon cells) and nematocysts (venomous harpoon cells). The tentacles then bend to push larger prey into the mouth, while smaller, plankton-size prey, is moved by cilia to the tip of the tentacles which are then inserted into the mouth. The mouth can stretch to accommodate large items, and in some species, the lips may extend to help receive the prey. The pharynx then grasps the prey, which is mixed with mucus and slowly swallowed by peristalsis and ciliary action. When the food reaches the coelenteron, extracellular digestion is initiated by the discharge of the septa-based nematocysts and the release of enzymes. The partially digested food fragments are circulated in the coelenteron by cilia, and from here they are taken up by phagocytosis by the gastrodermal cells that line the cavity.[2]
Most anthozoans supplement their predation by incorporating into their tissues certain unicellular, photosynthetic organisms known as zooxanthellae (or zoochlorellae in a few instances); many fulfil the bulk of their nutritional requirements in this way. In this symbiotic relationship, the zooxanthellae benefit by using nitrogenous waste and carbon dioxide produced by the host while the cnidarian gains photosynthetic capability and increased production of calcium carbonate, a substance of great importance to stony corals.[17] The presence of zooxanthellae is not a permanent relationship. Under some circumstances, the symbionts can be expelled, and other species may later move in to take their place. The behaviour of the anthozoan can also be affected, with it choosing to settle in a well lit spot, and competing with its neighbours for light to allow photosynthesis to take place. Where an anthozoan lives in a cave or other dark location, the symbiont may be absent in a species that, in a sunlit location, normally benefits from one.[18] Anthozoans living at depths greater than 50 m (200 ft) are azooxanthellate because there is insufficient light for photosynthesis.[4]

With longitudinal, transverse and radial muscles, polyps are able to elongate and shorten, bend and twist, inflate and deflate, and extend and contract their tentacles. Most polyps extend to feed and contract when disturbed, often invaginating their oral discs and tentacles into the column. Contraction is achieved by pumping fluid out of the coelenteron, and reflation by drawing it in, a task performed by the siphonoglyphs in the pharynx which are lined with beating cilia. Most anthozoans adhere to the substrate with their pedal discs but some are able to detach themselves and move about, while others burrow into the sediment. Movement may be a passive drifting with the currents or in the case of sea anemones, may involve creeping along a surface on their base.[2]
Gas exchange and excretion is accomplished by diffusion through the tentacles and internal and external body wall, aided by the movement of fluid being wafted along these surfaces by cilia. The sensory system consists of simple nerve nets in the gastrodermis and epidermis, but there are no specialised sense organs.[2]
Anthozoans exhibit great powers of regeneration; lost parts swiftly regrow and the sea anemone Aiptasia pallida can be vivisected in the laboratory and then returned to the aquarium where it will heal. They are capable of a variety of asexual means of reproduction including fragmentation, longitudinal and transverse fission and budding.[2] Sea anemones for example can crawl across a surface leaving behind them detached pieces of the pedal disc which develop into new clonal individuals. Anthopleura species divide longitudinally, pulling themselves apart, resulting in groups of individuals with identical colouring and patterning.[19] Transverse fission is less common, but occurs in Anthopleura stellula and Gonactinia prolifera, with a rudimentary band of tentacles appearing on the column before the sea anemone tears itself apart.[20] Zoanthids are capable of budding off new individuals.[21]

Most anthozoans are unisexual but some stony corals are hermaphrodite. The germ cells originate in the endoderm and move to the gastrodermis where they differentiate. When mature, they are liberated into the coelenteron and thence to the open sea, with fertilisation being external.[2] To make fertilisation more likely, corals emit vast numbers of gametes, and many species synchronise their release in relation to the time of day and the phase of the moon.[22]
The zygote develops into a planula larva which swims by means of cilia and forms part of the plankton for a while before settling on the seabed and metamorphosing into a juvenile polyp. Some planulae contain yolky material and others incorporate zooxanthellae, and these adaptations enable these larvae to sustain themselves and disperse more widely.[2] The planulae of the stony coral Pocillopora damicornis, for example, have lipid-rich yolks and remain viable for as long as 100 days before needing to settle.[23]
Ecology

Coral reefs are some of the most biodiverse habitats on earth, supporting large numbers of species of corals, fish, molluscs, worms, arthropods, starfish, sea urchins, other invertebrates and algae. Because of the photosynthetic requirements of the corals, they are found in shallow waters, and many of these fringe land masses.[24] With a three-dimensional structure, coral reefs are very productive ecosystems; they provide food for their inhabitants, hiding places of various sizes to suit many organisms, perching places, barriers to large predators and solid structures on which to grow. They are used as breeding grounds and as nurseries by many species of pelagic fish, and they influence the productivity of the ocean for miles around.[25] Anthozoans prey on animals smaller than they are and are themselves eaten by such animals as fish, crabs, barnacles, snails and starfish. Their habitats are easily disturbed by outside factors which unbalance the ecosystem. In 1989, the invasive crown-of-thorns starfish (Acanthaster planci) caused havoc in American Samoa, killing 90% of the corals in the reefs.[26]
Corals that grow on reefs are called hermatypic, with those growing elsewhere are known as ahermatypic. Most of the latter are azooxanthellate and live in both shallow and deep sea habitats. In the deep sea they share the ecosystem with soft corals, polychaete worms, other worms, crustaceans, molluscs and sponges. In the Atlantic Ocean, the cold-water coral Lophelia pertusa forms extensive deep-water reefs which support many other species.[27]
Other fauna, such as hydrozoa, bryozoa and brittle stars, often dwell among the branches of gorgonian and coral colonies.[28] The pygmy seahorse not only makes certain species of gorgonians its home, but closely resembles its host and is thus well camouflaged.[29] Some organisms have an obligate relationship with their host species. The mollusc Simnialena marferula is only found on the sea whip Leptogorgia virgulata, is coloured like it and has sequestered its defensive chemicals, and the nudibranch Tritonia wellsi is another obligate symbiont, its feathery gills resembling the tentacles of the polyps.[30]

A number of sea anemone species are commensal with other organisms. Certain crabs and hermit crabs seek out sea anemones and place them on their shells for protection, and fish, shrimps and crabs live among the anemone's tentacles, gaining protection by being in close proximity to the stinging cells. Some amphipods live inside the coelenteron of the sea anemone.[31] Despite their venomous cells, sea anemones are eaten by fish, starfish, worms, sea spiders and molluscs. The sea slug Aeolidia papillosa feeds on the aggregating anemone (Anthopleura elegantissima), accumulating the nematocysts for its own protection.[31]
Paleontology
Several extinct orders of corals from the Paleozoic era (~540–252 million years ago) are thought to be close to the ancestors of modern Scleractinia:[32][33]
- Numidiaphyllida †
- Kilbuchophyllida †
- Heterocorallia †
- Rugosa †
- Heliolitida †
- Tabulata †
- Cothoniida †
- Tabuloconida †
These are all corals and correspond to the fossil record time line. With readily-preserved hard calcareous skeletons, they comprise the majority of Anthozoan fossils.
Interactions with humans
Coral reefs and shallow marine environments are threatened, not only by natural events and increased sea temperatures, but also by such man-made problems as pollution, sedimentation and destructive fishing practices. Pollution may be the result of run-off from the land of sewage, agricultural products, fuel or chemicals. These may directly kill or injure marine life, or may encourage the growth of algae that smother native species, or form algal blooms with wide-ranging effects. Oil spills at sea can contaminate reefs, and also affect the eggs and larva of marine life drifting near the surface.[34]
Corals are collected for the aquarium trade, and this may be done with little care for the long-term survival of the reef. Fishing among reefs is difficult and trawling does much mechanical damage. In some parts of the world explosives are used to dislodge fish from reefs, and cyanide may be used for the same purpose; both practices not only kill reef inhabitants indiscriminately but also kill or damage the corals, sometimes stressing them so much that they expel their zooxanthellae and become bleached.[34]
Deep water coral habitats are also threatened by human activities, particularly by indiscriminate trawling. These ecosystems have been little studied, but in the perpetual darkness and cold temperatures, animals grow and mature slowly and there are relatively fewer fish worth catching than in the sunlit waters above. To what extent deep-water coral reefs provide a safe nursery area for juvenile fish has not been established, but they may be important for many cold-water species.[35]
References
- ↑ "Anthozoa: Etymology". Fine Dictionary. http://www.finedictionary.com/Anthozoa.html.
- ↑ 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 2.11 2.12 2.13 2.14 2.15 2.16 2.17 2.18 2.19 2.20 2.21 2.22 2.23 2.24 2.25 Ruppert, Edward E.; Fox, Richard, S.; Barnes, Robert D. (2004). Invertebrate Zoology (7th ed.). Cengage Learning. pp. 112–148. ISBN 978-81-315-0104-7.
- ↑ Crowther, A.L. (2011). "Class Anthozoa Ehrenberg, 1834". in Z.-Q. Zhang. Animal biodiversity: an outline of higher-level classification and survey of taxonomic richness. 3148. 19–23. doi:10.11646/zootaxa.3148.1.5. http://mapress.com/zootaxa/2011/f/zt03148p023.pdf.
- ↑ 4.0 4.1 Woodley, Cheryl M.; Downs, Craig A.; Bruckner, Andrew W.; Porter, James W.; Galloway, Sylvia B. (2016). Diseases of Coral. John Wiley & Sons. p. 416. ISBN 978-0-8138-2411-6. https://books.google.com/books?id=U8gbCQAAQBAJ&pg=PA416.
- ↑ 5.0 5.1 Stampar, S.N.; Maronna, M.M.; Kitahara, M.V.; Reimer, J.D.; Morandini, A.C. (March 2014). "Fast-Evolving Mitochondrial DNA in Ceriantharia: A Reflection of Hexacorallia Paraphyly?". PLOS ONE 9 (1): e86612. doi:10.1371/journal.pone.0086612. PMID 24475157. Bibcode: 2014PLoSO...986612S.
- ↑ 6.0 6.1 Daly, M.; Brugler, M.P.; Cartwright, P.; Collins, A.G.; Dawson, M.N.; Fautin, D.G.; France, S.C.; McFadden, C.S. et al. (2007). "The phylum Cnidaria: A review of phylogenetic patterns and diversity 300 years after Linnaeus". Zootaxa 1668: 1–766. doi:10.5281/zenodo.180149. http://www.nhm.ku.edu/inverts/pdf/daly%20et%20al%20Zootaxa.pdf.
- ↑ Chen, C. A.; D. M. Odorico; M. ten Lohuis; J. E. N. Veron; D. J. Miller (June 1995). "Systematic relationships within the Anthozoa (Cnidaria: Anthozoa) using the 5'-end of the 28S rDNA". Molecular Phylogenetics and Evolution 4 (2): 175–183. doi:10.1006/mpev.1995.1017. PMID 7663762.
- ↑ Hoeksema, Bert (2013). "Anthozoa". WoRMS. World Register of Marine Species. http://www.marinespecies.org/aphia.php?p=taxdetails&id=1292.
- ↑ 9.0 9.1 Taylor, Paul D.; Lewis, David N. (2007). Fossil Invertebrates. Harvard University Press. p. 25. ISBN 978-0-674-02574-5. https://books.google.com/books?id=7kPwZ2LeSAoC&pg=PA25.
- ↑ 10.0 10.1 Barnes, Robert D. (1982). Invertebrate Zoology. Holt-Saunders International. pp. 168–169. ISBN 978-0-03-056747-6.
- ↑ Fabricius, Katharina; Alderslade, Philip (2001). Soft Corals and Sea Fans: A Comprehensive Guide to the Tropical Shallow Water Genera of the Central-West Pacific, the Indian Ocean and the Red Sea. Australian Institute of Marine Science. p. 6. ISBN 978-0-642-32210-4. https://books.google.com/books?id=vYYWAQAAIAAJ.
- ↑ Williams, G.C. (2011). "The global diversity of sea pens (Cnidaria: Octocorallia: Pennatulacea)". PLOS ONE 6 (7): e22747. doi:10.1371/journal.pone.0022747. PMID 21829500. Bibcode: 2011PLoSO...622747W.
- ↑ 13.0 13.1 Goffredo, Stefano; Dubinsky, Zvy (2016). The Cnidaria, Past, Present and Future: The world of Medusa and her sisters. Springer International Publishing. p. 66. ISBN 978-3-319-31305-4. https://books.google.com/books?id=RdYADQAAQBAJ&pg=PA66.
- ↑ 14.0 14.1 Fautin, Daphne G.; Westfall, Jane A.; Cartwright, Paulyn; Daly, Marymegan; Wyttenbach, Charles R. (2007). Coelenterate Biology 2003: Trends in Research on Cnidaria and Ctenophora. Springer Science & Business Media. p. 261. ISBN 978-1-4020-2762-8. https://books.google.com/books?id=vbySjNMSYuwC&pg=PA261.
- ↑ "Corals: How do stony corals grow? What forms do they take?". National Oceanic and Atmospheric Administration. 25 March 2008. http://oceanservice.noaa.gov/education/kits/corals/coral03_growth.html.
- ↑ "Introduction to the Octocorallia". University of California Museum of Paleontology. http://www.ucmp.berkeley.edu/cnidaria/octocorallia.html.
- ↑ "Contribution to the BUFUS Newsletter, Field excursion to Milne Bay Province - Papua New Guinea, Madl and Yip 2000". http://biophysics.sbg.ac.at/png/png3.htm.
- ↑ Light, Sol Felty (2007). The Light and Smith Manual: Intertidal Invertebrates from Central California to Oregon. University of California Press. p. 176. ISBN 978-0-520-23939-5. https://books.google.com/books?id=64jgZ1CfmB8C&pg=PA176.
- ↑ Geller, Jonathan B.; Fitzgerald, Laurie J.; King, Chad E. (2005). "Fission in Sea Anemones: Integrative Studies of Life Cycle Evolution1". Integrative and Comparative Biology 45 (4): 615–622. doi:10.1093/icb/45.4.615. PMID 21676808.
- ↑ The Cnidaria, Past, Present and Future: The world of Medusa and her sisters. Springer International Publishing. 2016. p. 240. ISBN 978-3-319-31305-4. https://books.google.com/books?id=RdYADQAAQBAJ&pg=PA240.
- ↑ Carefoot, Tom. "Learn about Sea Anemones and Relatives: Reproduction: Asexual". A Snail's Odyssey. http://www.asnailsodyssey.com/LEARNABOUT/ANEMONE/anemAsex.php.
- ↑ "Corals: How do corals reproduce?". National Oceanic and Atmospheric Administration. 25 March 2008. http://oceanservice.noaa.gov/education/kits/corals/coral06_reproduction.html.
- ↑ Richmond, R. H. (1987). "Energetics, competency, and long-distance dispersal of planula larvae of the coral Pocillopora damicornis". Marine Biology 93 (4): 527–533. doi:10.1007/BF00392790.
- ↑ "Corals: Importance of coral reefs". National Oceanic and Atmospheric Administration. 25 March 2008. http://oceanservice.noaa.gov/education/kits/corals/coral07_importance.html.
- ↑ Sorokin, Yuri I. (2013). Coral Reef Ecology. Springer Science & Business Media. pp. 1–3. ISBN 978-3-642-80046-7. https://books.google.com/books?id=hKvrCAAAQBAJ&pg=PA1.
- ↑ "Corals: Natural Threats to Coral Reefs". National Oceanic and Atmospheric Administration. 25 March 2008. http://oceanservice.noaa.gov/education/kits/corals/coral08_naturalthreats.html.
- ↑ Roberts, J. Murray (2009). Cold-Water Corals: The Biology and Geology of Deep-Sea Coral Habitats. Cambridge University Press. p. 152. ISBN 978-0-521-88485-3. https://books.google.com/books?id=L6X6UW7ZszIC&pg=PA152.
- ↑ Haywood, Martyn; Sue Wells (1989). The Manual of Marine Invertebrates. Tetra Press:Salamander Books Ltd.. p. 208. ISBN 978-3-89356-033-2. https://archive.org/details/manualofmarinein0000hayw.
- ↑ Agbayani, Eli. "Hippocampus bargibanti, Pygmy seahorse". FishBase. http://www.fishbase.org/Summary/SpeciesSummary.php?id=53790.
- ↑ Ruppert, Edward E.; Richard S. Fox (1988). Seashore animals of the Southeast: a guide to common shallow-water invertebrates of the Southeastern Atlantic Coast. University of South Carolina Press. p. 124. ISBN 9780872495357. https://archive.org/details/seashoreanimalso00edwa.
- ↑ 31.0 31.1 Van-Praët, M. (1985). Advances in Marine Biology. Academic Press. pp. 92–93. ISBN 978-0-08-057945-0. https://books.google.com/books?id=xkKOLOunEfIC&pg=PA92.
- ↑ Oliver W.A. Jr. (1996). "Origins and relationships of Paleozoic coral groups and the origin of the Scleractinia". in Stanley, G.D.J.. Paleobiology and Biology of Corals. 1. Columbus, Ohio: The Paleontological Society. 107–134. doi:10.1017/S1089332600000073.
- ↑ Kotrc, Ben (2005). "Anthozoa: Subgroups". Fossil Groups. University of Bristol. http://palaeo.gly.bris.ac.uk/Palaeofiles/Fossilgroups/anthozoa/subgroups.html.
- ↑ 34.0 34.1 "Corals: Anthropogenic threats to corals". National Oceanic and Atmospheric Administration. 25 March 2008. http://oceanservice.noaa.gov/education/kits/corals/coral09_humanthreats.html.
- ↑ Roberts, J. Murray (2009). Cold-Water Corals: The Biology and Geology of Deep-Sea Coral Habitats. Cambridge University Press. p. 163. ISBN 978-0-521-88485-3. https://books.google.com/books?id=L6X6UW7ZszIC&pg=PA163.
External links
Wikidata ☰ Q28524 entry
 |
 KSF
KSF

