-
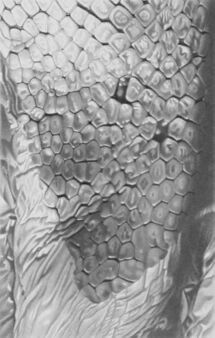
Mature female of Onykia ingens (38.4 cm ML)
-
Piece of ventral mantle skin from O. ingens
-
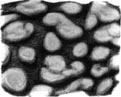
Large mature female of Onykia robsoni (88.5 cm ML) weighing 11.1 kg
-
Closeup of the dermal warts from the same O. robsoni specimen
-
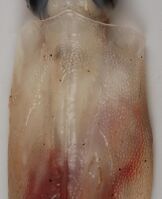
Dorsal mantle of Slosarczykovia circumantarctica (12.2 cm ML)
-
Ocythoe tuberculata with ridges and tubercles visible on mantle
Cephalopod dermal structures
Topic: Biology
 From HandWiki - Reading time: 6 min
From HandWiki - Reading time: 6 min
Cephalopods exhibit various dermal structures on their mantles and other parts. These may take the form of conspicuous warts, cushions, papillae or scales, though in many species they are microscopic tubercles.[4] The most elaborate forms are found among the oceanic squid of the order Teuthida.[1][5]
Morphology and composition
Most cephalopod dermal structures take the form of tubercles, and these are the only cartilaginous dermal structures (the various "dermal cushions" being composed of other forms of connective tissue). All three main types of cartilage found in vertebrates are represented among the different squid species: hyaline, elastic and fibrocartilage. Tubercles of hyaline cartilage are primarily associated with cranchiid or glass squid.[1]
The vast majority of cephalopod dermal structures have a thin, overlying epidermal layer, though this is often damaged or missing in captured specimens.[1]
| Species | Family | Structure | Shape | Size in mm (ML in mm) | Tissue | Epidermis over structure |
|---|---|---|---|---|---|---|
| Asperoteuthis acanthoderma | Chiroteuthidae | Tubercles | Conical | 1.0 × 0.4 (144) | Hyaline-like cartilage | Yes |
| Cranchia scabra | Cranchiidae | Tubercles | Round, triangular, or rectangular bases with 2–5 projections | 0.4–0.8 × 0.2–0.4 (94) | Hyaline cartilage | Yes |
| Histioteuthis meleagroteuthis | Histioteuthidae | Tubercles | Knob-like | from mantle ridge: 0.4–0.8 × 1.3–1.9; from arm ridge: 0.6–2.2 × 0.7–2.3 (38) | Elastic cartilage | Yes |
| Leachia cyclura | Cranchiidae | Tubercles | Small conical to large complex with numerous conical projections | 0.3–0.4 × 0.1–0.2 (55) | Hyaline cartilage | Yes |
| Lepidoteuthis grimaldii | Lepidoteuthidae | Dermal cushions ("scales") | Rhomboid to hexagonal | 2.0 × 0.5 (180) | Connective tissue in honeycomb arrangement | Yes |
| Liocranchia reinhardti | Cranchiidae | Tubercles | Conical | 0.2–0.3 × 0.15–0.2 (29) | Hyaline cartilage | Yes |
| Mastigoteuthis cordiformis | Mastigoteuthidae | Tubercles | Conical | 0.2 × 0.3 (87) | Elastic or fibrocartilage | Yes |
| Mastigoteuthis hjorti | Mastigoteuthidae | Tubercles | Conical | 0.1–0.2 × 0.05–0.1 (93) | Elastic or fibrocartilage | Yes |
| Pholidoteuthis adami | Pholidoteuthidae | Dermal cushions | Round to pentagonal | 0.5 × 0.3 (300) | Connective tissue in honeycomb arrangement | Yes |
| Pholidoteuthis massyae | Pholidoteuthidae | Tubercles and papillae | Roundish | 0.3 × 0.15 (100) | Dense connective tissue with chondrocytes; elastic cartilage | No; acellular cuticle |
Other cephalopods with prominent dermal structures include: Brachioteuthis spp.; Galiteuthis glacialis (which has round tubercles); Histioteuthis meleagroteuthis and Histioteuthis miranda (tuberculate ridges); Mastigoteuthis danae (large tubercles in advanced paralarvae);[6] Onykia aequatorialis, Onykia ingens, Onykia lonnbergii, and Onykia robsoni (irregular warts);[7] Slosarczykovia circumantarctica (fibrous integumental net);[8] and all members of the subfamily Cranchiinae (strips of cartilaginous tubercles in various arrangements).[1] Among octopuses, Ocythoe tuberculata is noted for the tubercles and ridges on its mantle.[1]
Function


Different cephalopod dermal structures are hypothesised to play roles in buoyancy, locomotion, and even pseudoskeletal support.[1]
Buoyancy aid
Two fundamentally different buoyancy mechanisms associated with dermal structures have been proposed.[1]
Buoyancy vest
The mantle of Cranchia scabra is covered in multi-pointed cartilaginous tubercles.[9][10] An anti-predator function has been proposed in the past, but this is thought unlikely given the small size and transparent nature of the tubercles. The tubercles of this species are covered by a thin, epidermal sheath that is often lost during capture. It has been speculated that in the live animal the interstitial space is filled with a buoyant fluid (likely an ammonium chloride solution) and acts as a "buoyancy vest". The hard tubercles may serve to maintain the shape of this structure. It has been estimated that in a C. scabra measuring 10 cm in mantle length (ML), the buoyancy vest could contribute an additional 4% to the animal's total buoyant fluid (most of the remainder being located in the coelom), probably sufficient to achieve neutral buoyancy.[1]
A similar mechanism may be utilised by the much larger Galiteuthis glacialis, which has a very similar combination of tubercles and overlying epidermal sheath.[1]


Fluid-filled dermal cushions
The overlapping "scales" of Lepidoteuthis grimaldii are actually dermal cushions with a vacuolate internal structure that are continuous with a similarly vacuolate underlying layer of mantle tissue.[1][11] Ammonium ions (NH4+) are present in the mantle of this species at a measured concentration of 172 mM.[12] Structurally very similar (though non-overlapping) dermal cushions are found in Pholidoteuthis adami.[1][13][14] It has been proposed that these two species achieve buoyancy by means of the fluid stored in their vacuolate dermal cushions and upper mantle layer.[1] Given their spongy form, these cushions may also play a secondary protective role.[1]
Drag reduction

The complex[15] dermal structures of Pholidoteuthis massyae may play a role in reducing hydrodynamic drag. More specifically, they may be involved in maintaining laminar flow by preventing or delaying boundary layer separation along the mantle.[1]
It is possible that a similar locomotory mechanism is present in Mastigoteuthis cordiformis and Mastigoteuthis hjorti, though the small size of the tubercles in these species may preclude such a function.[1]
Pseudoskeletal support
In the cranchiids Leachia cyclura and Liocranchia reinhardti, the dermal tubercles are not distributed throughout the mantle but arranged in discrete cartilaginous bands. A role in buoyancy control is therefore unlikely. One possibility is that these rigid bands play a pseudoskeletal role, maintaining the shape of parts of the mantle during swimming contractions or providing attachment points for mantle muscles or visceral tissue (such as the septum covering the coelom).[1]
The very dense tuberculate ridges found on the arms and dorsal mantle of Histioteuthis meleagroteuthis may similarly provide insertion points for muscles, and are probably most important in juvenile animals, which lack well-developed musculature.[1] In the two other Histioteuthis species with tuberculate ridges—H. meleagroteuthis and H. miranda—these structures likely have the same function.[1]
Unknown function
The mantle of Asperoteuthis acanthoderma is covered in minute, widely spaced tubercles of hyaline-like cartilage. In a 1990 study of dermal structures in squid, Clyde F. E. Roper and C. C. Lu wrote that they were "unable to suggest a function" for the tubercles of this species, but that due to their small size and spacing they were unlikely to be involved in buoyancy or locomotion.[1]
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 1.14 1.15 1.16 1.17 1.18 1.19 1.20 Roper, C.F.E. & C.C. Lu (1990). "Comparative morphology and function of dermal structures in oceanic squids (Cephalopoda).". http://www.sil.si.edu/SmithsonianContributions/Zoology/pdf_hi/SCTZ-0493.pdf. Smithsonian Contributions to Zoology, Number 493: 1–40.
- ↑ Clarke, M.R. (1960). Lepidoteuthis grimaldii—a squid with scales. Nature 188: 955–956 doi:10.1038/188955a0
- ↑ Clarke, M.R. & G.E. Maul (1962). A description of the "scaled" squid Lepidoteuthis grimaldi Joubin 1895. Proceedings of the Zoological Society of London 139(1): 97–118. doi:10.1111/j.1469-7998.1962.tb01824.x
- ↑ Young, R.E., M. Vecchione & K.M. Mangold (2001). Cephalopod Mantle Armature. Tree of Life Web Project.
- ↑ Roper, C.F.E. & C.C. Lu (1989). "Systematic status of Lepidoteuthis, Pholidoteuthis and Tetronychoteuthis (Cephalopoda: Oegopsida).". http://si-pddr.si.edu/jspui/bitstream/10088/11019/1/iz_Roper-1989.pdf. Proceedings of the Biological Society of Washington 102(3): 805–807.
- ↑ Vecchione, M. & R.E. Young (2007). Mastigoteuthis danae (Joubin, 1933). Tree of Life Web Project.
- ↑ Bolstad, K.S.R., M. Vecchione & R.E. Young (2011). Onykia Lesueur, 1821. Tree of Life Web Project.
- ↑ Lipinski, M. & R.E. Young (2011). Slosarczykovia. Tree of Life Web Project.
- ↑ Person, P. (1969). Cartilaginous dermal scales in cephalopods. Science 164(3886): 1404–1405. doi:10.1126/science.164.3886.1404
- ↑ Dilly, P.N. & M. Nixon (1976). The dermal tubercles of Cranchia scabra (Mollusca, Cephalopoda); surface structure and development. Journal of Zoology 179(3): 291–295. doi:10.1111/j.1469-7998.1976.tb02297.x
- ↑ Young, R.E. & M. Vecchione (1999). Lepidoteuthis grimaldii: Dermal Cushions. Tree of Life Web Project.
- ↑ Lipiński, M. & K. Turoboyski (1983). The ammonium content in the tissues of selected species of squid (Cephalopoda: Teuthoidea). Journal of Experimental Marine Biology and Ecology 69(2): 145–150. doi:10.1016/0022-0981(83)90064-3
- ↑ Goldman, D.A. (1995). A juvenile of the scaled squid, Pholidoteuthis adami Voss, 1956 (Cephalopoda: Oegopsida), from the Florida Keys. Proceedings of the Biological Society of Washington 108(1): 136–146.
- ↑ Young, R.E. & M. Vecchione (1999). Pholidoteuthis adami Dermal Cushions. Tree of Life Web Project.
- ↑ Young, R.E. & M. Vecchione (1999). Pholidoteuthis massyae Mantle Dermal Cushions. Tree of Life Web Project.
 |
 KSF
KSF