Liquid breathing
Topic: Biology
 From HandWiki - Reading time: 20 min
From HandWiki - Reading time: 20 min
| Liquid breathing | |
|---|---|
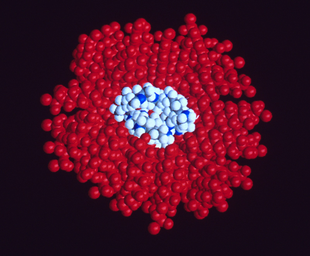 Computer-generated model of perflubron and gentamicin molecules in liquid suspension for pulmonary administration | |
| MeSH | D021061 |
Liquid breathing is a form of respiration in which a normally air-breathing organism breathes an oxygen-rich liquid (such as a perfluorocarbon), rather than breathing air, by selecting a liquid that can hold a large amount of oxygen and is capable of CO2 gas exchange.[1]
This requires certain physical properties such as respiratory gas solubility, density, viscosity, vapor pressure, and lipid solubility which some perfluorochemicals (PFCs) have.[2] Thus, it is critical to choose the appropriate PFC for a specific biomedical application, such as liquid ventilation, drug delivery or blood substitutes. The physical properties of PFC liquids vary substantially; however, the one common property is their high solubility for respiratory gases. In fact, these liquids carry more oxygen and carbon dioxide than blood.[3]
In theory, liquid breathing could assist in the treatment of patients with severe pulmonary or cardiac trauma, especially in pediatric cases. Liquid breathing has also been proposed for use in deep diving[4][5][6] and space travel.[7][8] Despite some recent advances in liquid ventilation, a standard mode of application has not yet been established.
Approaches
| Gas solubility | |
| Oxygen | 33–66 mL / 100 mL PFC |
| Carbon dioxide | 140–166 mL / 100 mL PFC |
| Vapor pressure | 0.2–400 torr |
| Density | 1.58–2.0 g/mL |
| Viscosity | 0.8–8.0 cSt |
Because liquid breathing is still a highly experimental technique, there are several proposed approaches.
Total liquid ventilation
Although total liquid ventilation (TLV) with completely liquid-filled lungs can be beneficial,[9] the complex liquid-filled tube system required is a disadvantage compared to gas ventilation—the system must incorporate a membrane oxygenator, heater, and pumps to deliver to, and remove from the lungs tidal volume aliquots of conditioned perfluorocarbon (PFC). One research group led by Thomas H. Shaffer has maintained that with the use of microprocessors and new technology, it is possible to maintain better control of respiratory variables such as liquid functional residual capacity and tidal volume during TLV than with gas ventilation.[2][10][11][12] Consequently, the total liquid ventilation necessitates a dedicated liquid ventilator similar to a medical ventilator except that it uses a breathable liquid. Many prototypes are used for animal experimentation, but experts recommend continued development of a liquid ventilator toward clinical applications.[13] Specific preclinical liquid ventilator (Inolivent) is currently under joint development in Canada and France .[14] The main application of this liquid ventilator is the ultra-fast induction of therapeutic hypothermia after cardiac arrest. This has been demonstrated to be more protective than slower cooling method after experimental cardiac arrest.[15]
Partial liquid ventilation
In contrast, partial liquid ventilation (PLV) is a technique in which a PFC is instilled into the lung to a volume approximating functional residual capacity (approximately 40% of total lung capacity). Conventional mechanical ventilation delivers tidal volume breaths on top of it. This mode of liquid ventilation currently seems technologically more feasible than total liquid ventilation, because PLV could utilise technology currently in place in many neonatal intensive-care units (NICU) worldwide.
The influence of PLV on oxygenation, carbon dioxide removal and lung mechanics has been investigated in several animal studies using different models of lung injury.[16] Clinical applications of PLV have been reported in patients with acute respiratory distress syndrome (ARDS), meconium aspiration syndrome, congenital diaphragmatic hernia and respiratory distress syndrome (RDS) of neonates. In order to correctly and effectively conduct PLV, it is essential to
- properly dose a patient to a specific lung volume (10–15 ml/kg) to recruit alveolar volume
- redose the lung with PFC liquid (1–2 ml/kg/h) to oppose PFC evaporation from the lung.
If PFC liquid is not maintained in the lung, PLV can not effectively protect the lung from biophysical forces associated with the gas ventilator.
New application modes for PFC have been developed.[17]
Partial liquid ventilation (PLV) involves filling the lungs with a liquid. This liquid is a perfluorocarbon such as perflubron (brand name Liquivent). The liquid has some unique properties. It has a very low surface tension, similar to the surfactant substances produced in the lungs to prevent the alveoli from collapsing and sticking together during exhalation. It also has a high density, oxygen readily diffuses through it, and it may have some anti-inflammatory properties. In PLV, the lungs are filled with the liquid, the patient is then ventilated with a conventional ventilator using a protective lung ventilation strategy. The hope is that the liquid will help the transport of oxygen to parts of the lung that are flooded and filled with debris, help remove this debris and open up more alveoli improving lung function. The study of PLV involves comparison to protocolized ventilator strategy designed to minimize lung damage.[18][19]
PFC vapor
Vaporization of perfluorohexane with two anesthetic vaporizers calibrated for perfluorohexane has been shown to improve gas exchange in oleic acid-induced lung injury in sheep.[20]
Predominantly PFCs with high vapor pressure are suitable for vaporization.
Aerosol-PFC
With aerosolized perfluorooctane, significant improvement of oxygenation and pulmonary mechanics was shown in adult sheep with oleic acid-induced lung injury.
In surfactant-depleted piglets, persistent improvement of gas exchange and lung mechanics was demonstrated with Aerosol-PFC.[21] The aerosol device is of decisive importance for the efficacy of PFC aerosolization, as aerosolization of PF5080 (a less purified FC77) has been shown to be ineffective using a different aerosol device in surfactant-depleted rabbits. Partial liquid ventilation and Aerosol-PFC reduced pulmonary inflammatory response.[22]
Human usage
Medical treatment
The most promising area for the use of liquid ventilation is in the field of pediatric medicine.[23][24][25] The first medical use of liquid breathing was treatment of premature babies[26][27][28][29] and adults with acute respiratory distress syndrome (ARDS) in the 1990s. Liquid breathing was used in clinical trials after the development by Alliance Pharmaceuticals of the fluorochemical perfluorooctyl bromide, or perflubron for short. Current methods of positive-pressure ventilation can contribute to the development of lung disease in pre-term neonates, leading to diseases such as bronchopulmonary dysplasia. Liquid ventilation removes many of the high pressure gradients responsible for this damage. Furthermore, perfluorocarbons have been demonstrated to reduce lung inflammation,[30][31][32] improve ventilation-perfusion mismatch and to provide a novel route for the pulmonary administration of drugs.[30][33][34]
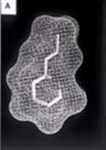
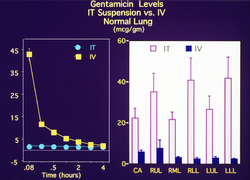
In order to explore drug delivery techniques that would be useful for both partial and total liquid ventilation, more recent studies have focused on PFC drug delivery using a nanocrystal suspension. The first image is a computer model of a PFC liquid (perflubron) combined with gentamicin molecules.
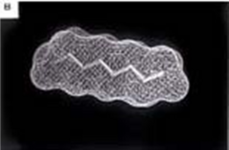
The second image shows experimental results comparing both plasma and tissue levels of gentamicin after an intratracheal (IT) and intravenous (IV) dose of 5 mg/kg in a newborn lamb during gas ventilation. Note that the plasma levels of the IV dose greatly exceed the levels of the IT dose over the 4 hour study period; whereas, the lung tissue levels of gentamicin when delivered by an intratracheal (IT) suspension, uniformly exceed the intravenous (IV) delivery approach after 4 hours. Thus, the IT approach allows more effective delivery of the drug to the target organ while maintaining a safer level systemically. Both images represent the in-vivo time course over 4 hours. Numerous studies have now demonstrated the effectiveness of PFC liquids as a delivery vehicle to the lungs.[35][36][37][38][34][39][33][40][30][41]
Clinical trials with premature infants and adults have been conducted.[42] Since the safety of the procedure and the effectiveness were apparent from an early stage, the US Food and Drug Administration (FDA) gave the product "fast track" status (meaning an accelerated review of the product, designed to get it to the public as quickly as is safely possible) due to its life-saving potential. Clinical trials showed that using perflubron with ordinary ventilators improved outcomes as much as using high frequency oscillating ventilation (HFOV). But because perflubron was not better than HFOV, the FDA did not approve perflubron, and Alliance is no longer pursuing the partial liquid ventilation application. Whether perflubron would improve outcomes when used with HFOV or has fewer long-term consequences than HFOV remains an open question.
In 1996 Mike Darwin and Steven B. Harris proposed using cold liquid ventilation with perfluorocarbon to quickly lower the body temperature of victims of cardiac arrest and other brain trauma to allow the brain to better recover.[43] The technology came to be called gas/liquid ventilation (GLV), and was shown able to achieve a cooling rate of 0.5 °C per minute in large animals.[44] It has not yet been tried in humans.
Most recently, hypothermic brain protection has been associated with rapid brain cooling. In this regard, a new therapeutic approach is the use of intranasal perfluorochemical spray for preferential brain cooling.[45] The nasopharyngeal (NP) approach is unique for brain cooling due to anatomic proximity to the cerebral circulation and arteries. Based on preclinical studies in adult sheep, it was shown that independent of region, brain cooling was faster during NP-perfluorochemical versus conventional whole body cooling with cooling blankets. To date, there have been four human studies including a completed randomized intra-arrest study (200 patients).[46][47] Results clearly demonstrated that prehospital intra-arrest transnasal cooling is safe, feasible and is associated with an improvement in cooling time.
Proposed uses
Diving
Gas pressure increases with depth, rising 1 bar (14.5 psi (100 kPa)) every 10 meters to over 1,000 bar at the bottom of the Mariana Trench. Diving becomes more dangerous as depth increases, and deep diving presents many hazards. All surface-breathing animals are subject to decompression sickness, including aquatic mammals[48] and free-diving humans. Breathing at depth can cause nitrogen narcosis and oxygen toxicity. Holding the breath while ascending after breathing at depth can cause air embolisms, burst lung, and collapsed lung.
Special breathing gas mixes such as trimix or heliox reduce the risk of nitrogen narcosis but do not eliminate it. Heliox further eliminates the risk of nitrogen narcosis but introduces the risk of helium tremors below about 500 feet (150 m). Atmospheric diving suits maintain body and breathing pressure at 1 bar, eliminating most of the hazards of descending, ascending, and breathing at depth. However, the rigid suits are bulky, clumsy, and very expensive.
Liquid breathing offers a third option,[4][49] promising the mobility available with flexible dive suits and the reduced risks of rigid suits. With liquid in the lungs, the pressure within the diver's lungs could accommodate changes in the pressure of the surrounding water without the huge partial pressure gas exposures required when the lungs are filled with gas. Liquid breathing would not result in the saturation of body tissues with high pressure nitrogen or helium that occurs with the use of non-liquids, thus would reduce or remove the need for slow decompression.
A significant problem, however, arises from the high viscosity of the liquid and the corresponding reduction in its ability to remove CO2.[4][50] All uses of liquid breathing for diving must involve total liquid ventilation (see above). Total liquid ventilation, however, has difficulty moving enough liquid to carry away CO2, because no matter how great the total pressure is, the amount of partial CO2 gas pressure available to dissolve CO2 into the breathing liquid can never be much more than the pressure at which CO2 exists in the blood (about 40 mm of mercury (Torr)).[50]
At these pressures, most fluorocarbon liquids require about 70 mL/kg minute-ventilation volumes of liquid (about 5 L/min for a 70 kg adult) to remove enough CO2 for normal resting metabolism.[51] This is a great deal of fluid to move, particularly as liquids are more viscous and denser than gases, (for example water is about 850 times the density of air[52]). Any increase in the diver's metabolic activity also increases CO2 production and the breathing rate, which is already at the limits of realistic flow rates in liquid breathing.[4][53][54] It seems unlikely that a person would move 10 liters/min of fluorocarbon liquid without assistance from a mechanical ventilator, so "free breathing" may be unlikely. However, it has been suggested that a liquid breathing system could be combined with a CO2 scrubber connected to the diver's blood supply; a US patent has been filed for such a method.[55][56]
Space travel
Liquid immersion provides a way to reduce the physical stress of G forces. Forces applied to fluids are distributed as omnidirectional pressures. Because liquids cannot be practically compressed, they do not change density under high acceleration such as performed in aerial maneuvers or space travel. A person immersed in liquid of the same density as tissue has acceleration forces distributed around the body, rather than applied at a single point such as a seat or harness straps. This principle is used in a new type of G-suit called the Libelle G-suit, which allows aircraft pilots to remain conscious and functioning at more than 10g acceleration by surrounding them with water in a rigid suit.[57]
Acceleration protection by liquid immersion is limited by the differential density of body tissues and immersion fluid, limiting the utility of this method to about 15g to 20g.[58] Extending acceleration protection beyond 20g requires filling the lungs with fluid of density similar to water. An astronaut totally immersed in liquid, with liquid inside all body cavities, will feel little effect from extreme G forces because the forces on a liquid are distributed equally, and in all directions simultaneously. However effects will be felt because of density differences between different body tissues, so an upper acceleration limit still exists.
Liquid breathing for acceleration protection may never be practical because of the difficulty of finding a suitable breathing medium of similar density to water that is compatible with lung tissue. Perfluorocarbon fluids are twice as dense as water, hence unsuitable for this application.[3]
Examples in fiction
Literary works
- Alexander Beliaev's 1928 science fiction novel Amphibian Man is based on a scientist and a maverick surgeon, who makes his son, Ichthyander (etymology: "fish" + "man") a life-saving transplant – a set of shark gills. There is a film based on the novel.
- L. Sprague de Camp's 1938 short story "The Merman" hinges on an experimental process to make lungs function as gills, thus allowing a human being to "breathe" under water.
- Hal Clement's 1973 novel Ocean on Top portrays a small underwater civilization living in a 'bubble' of oxygenated fluid denser than seawater.
- Joe Haldeman's 1975 novel The Forever War describes liquid immersion and breathing in great detail as a key technology to allow space travel and combat with acceleration up to 50 G.
- In the Star Trek: The Next Generation novel The Children of Hamlin (1988) the crew of the Enterprise-D encounter an alien race whose ships contain a breathable liquid environment.
- Peter Benchley's 1994 novel White Shark centers around a Nazi scientist's experimental attempts to create an amphibious human, whose lungs are surgically modified to breathe underwater, and trained to reflexively do so after being flooded with a fluorocarbon solution.
- Judith and Garfield Reeves-Stevens' 1994 Star Trek novel Federation explains that before the invention of the inertial dampener, the stresses of high-G acceleration required starship pilots to be immersed in liquid-filled capsules, breathing an oxygen-rich saline solution to prevent their lungs from being crushed.
- Nicola Griffith's novel Slow River (1995) features a sex scene occurring within a twenty cubic foot silvery pink perflurocarbon pool, with the sensation described as "like breathing a fist".
- Ben Bova's novel Jupiter (2000) features a craft in which the crew are suspended in a breathable liquid that allows them to survive in the high-pressure environment of Jupiter's atmosphere.
- In Scott Westerfeld's sci-fi novel The Risen Empire (2003), the lungs of soldiers performing insertion from orbit are filled with an oxygen-rich polymer gel with embedded pseudo-alveoli and a rudimentary artificial intelligence.[59]
- The novel Mechanicum (2008) by Graham McNeill, Book 9 in the Horus Heresy book series, describes physically crippled Titan (gigantic war machine) pilots encased in nutrient fluid tanks. This allows them to continue operating beyond the limits normally imposed by the body.[60]
- In Liu Cixin's novel The Dark Forest (2008), the warships of humanity in the 23rd century flood their compartments with an oxygen-rich liquid called 'deep-sea acceleration fluid' to protect the crew against the forces of extreme acceleration that the ships undergo. Ships enter a 'deep-sea state' where the crew are immersed in the fluid and sedated before acceleration can commence.[61]
- In the 2009 novel The Lost Symbol by Dan Brown, Robert Langdon (the protagonist) is completely submerged in breathable liquid mixed with hallucinogenic chemicals and sedatives as a torture and interrogation technique by Mal'akh (the antagonist). He goes through a near death experience when he inhales the liquid and blacks out, losing control over his body, but is soon revived.
- In Greg van Eekhout's 2014 novel California Bones, two characters are put into tanks filled with liquid: "They were given no breathing apparatus, but the water in the tank was rich with perfluorocarbon, which carried more oxygen than blood."[62]
- In author A.L. Mengel's science fiction novel The Wandering Star (2016), several characters breathe oxygenated fluid during a dive to explore an underwater city. They submerge in high pressure "bubbles" filled with the perfluorocarbon fluid.
- In Tiamat's Wrath, a 2019 novel in The Expanse series by James S. A. Corey, The Laconian empire utilizes a ship with full-immersion liquid-breathing pods that allow the crew to undergo significantly increased g-forces. As powerful and fuel-efficient fusion engines in the series have made the only practical limitations of a ships' acceleration the survivability of the crew, this makes the ship the fastest in all of human-colonized space.
Films and television
- The aliens in the Gerry Anderson UFO series (1970-1971) use liquid-breathing spacesuits.
- The 1989 film The Abyss by James Cameron features a character using liquid breathing to dive thousands of feet without compressing. The Abyss also features a scene with a rat submerged in and breathing fluorocarbon liquid, filmed in real life.[63]
- In the 1995 anime Neon Genesis Evangelion, the cockpits of the titular mecha are filled with a fictional oxygenated liquid called LCL which is required for the pilot to mentally sync with an Evangelion, as well as providing direct oxygenation of their blood, and dampening the impacts from battle. Once the cockpit is flooded the LCL is ionized, bringing its density, opacity, and viscosity close to that of air. Protagonist Shinji Ikari notices that LCL smells like blood. It is eventually revealed that LCL is amniotic fluid created by the Evangelions' progenitor, Lilith.
- In the movie Mission to Mars (2000), a character is depicted as being immersed in apparent breathable fluid before a high-acceleration launch.
- In season 1, episode 13 of Seven Days (1998-2001) chrononaut Frank Parker is seen breathing a hyper-oxygenated perfluorocarbon liquid that is pumped through a sealed full body suit that he is wearing. This suit and liquid combination allow him to board a Russian submarine through open ocean at a depth of almost 1000 feet. Upon boarding the submarine he removes his helmet, expels the liquid from his lungs and is able to breathe air again.
- In an episode of the Adult Swim cartoon series Metalocalypse (2006-2013), the other members of the band submerge guitarist Toki in a "liquid oxygen isolation chamber" while recording an album in the Mariana Trench.
- In an episode of the Syfy Channel show Eureka (2006-2012), Sheriff Jack Carter is submerged in a tank of "oxygen rich plasma" to be cured of the effects of a scientific accident.
- In the anime series Aldnoah.Zero (2014-2015), episode 5 shows that Slaine Troyard was in a liquid-filled capsule when he crashed. Princess Asseylum witnessed the crash, helped him to get out of the capsule, then used CPR on him to draw out the liquid from his lungs.
Video games
- In the classic 1995 PC turn-based strategy game X-COM, "Aquanauts" fighting in deep ocean conditions breathe a dense oxygen-carrying fluid.
- In the EVE Online Universe (2003), pilots in capsules (escape pods that function as the control center for the spacecraft) breathe an oxygen rich, nano-saturated, breathable glucose-based suspension solution.[64]
See also
References
- ↑ GAEDEKE NORMS, M., RN, MSN, CCRN, CS, et al. Liquid Ventilation: It's Not Science Fiction Anymore. AACN Clin Issues Crit Care Nurs. 1994;5(3):246-254. Cited in: Your Journals@Ovid Full Text at http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=yrovftb&NEWS=N&AN=00002245-199408000-00004 .
- ↑ 2.0 2.1 Shaffer, Thomas H.; Wolfson, Marla R.; Clark, Leland C. (Oct 1992). "Liquid ventilation" (in en). Pediatric Pulmonology 14 (2): 102–109. doi:10.1002/ppul.1950140208. PMID 1437347.
- ↑ 3.0 3.1 Gabriel, Jerome L.; Miller, T. F.; Wolfson, Marla R.; Shaffer, Thomas H. (Nov 1996). "Quantitative Structure-Activity Relationships of Perfluorinated Hetero-Hydrocarbons as Potential Respiratory Media: Application to Oxygen Solubility, Partition Coefficient, Viscosity, Vapor Pressure, and Density" (in en). ASAIO Journal 42 (6): 968–973. doi:10.1097/00002480-199642060-00009. ISSN 1058-2916. PMID 8959271.
- ↑ 4.0 4.1 4.2 4.3 Kylstra JA (1977). The Feasibility of Liquid Breathing in Man.. Report to the US Office of Naval Research. Durham, NC: Duke University. http://archive.rubicon-foundation.org/4257. Retrieved 2008-05-05.
- ↑ "menfish". http://davidszondy.com/future/underwater/menfish.htm.
- ↑ Featured on the ABC television program That's Incredible, including a demonstration of a mouse surviving a prolonged dunking in perfluorocarbon.
- ↑ "Liquid Breathing - Medical uses". http://www.experiencefestival.com/a/Liquid_breathing_-_Medical_uses/id/1580110.
- ↑ Featured on the ABC television program That's Incredible. Cathy Lee Crosby describing diving and spaceflight applications. Voiceover with stock video.
- ↑ Wolfson, Marla R.; Hirschl, Ronald B.; Jackson, J Craig; Gauvin, France; Foley, David S.; Lamm, Wayne J. E.; Gaughan, John; Shaffer, Thomas H. (May 2008). "Multicenter Comparative Study of Conventional Mechanical Gas Ventilation to Tidal Liquid Ventilation in Oleic Acid Injured Sheep" (in en). ASAIO Journal 54 (3): 256–269. doi:10.1097/MAT.0b013e318168fef0. ISSN 1058-2916. PMID 18496275.
- ↑ Cox CA, Stavis RL. Wolfson MR, Shaffer TH; Stavis; Wolfson; Shaffer (2003). "Long-term tidal liquid ventilation in premature lambs: Physiologic, biochemical and histological correlates". Biol. Neonate 84 (3): 232–242. doi:10.1159/000072307. PMID 14504447.
- ↑ Libros, R.; Philips, C. M.; Wolfson, M. R.; Shaffer, T. H. (Sep 2000). "A perfluorochemical loss/restoration (L/R) system for tidal liquid ventilation". Biomedical Instrumentation & Technology 34 (5): 351–360. ISSN 0899-8205. PMID 11098391.
- ↑ Heckman, J. L.; Hoffman, J.; Shaffer, T. H.; Wolfson, M. R. (May 1999). "Software for real-time control of a tidal liquid ventilator". Biomedical Instrumentation & Technology 33 (3): 268–276. ISSN 0899-8205. PMID 10360217.
- ↑ Costantino, ML; Micheau, P; Shaffer, TH; Tredici, S et al. (2009). "Clinical design functions: Round table discussions on bioengineering of liquid ventilators". ASAIO J. 55 (3): 206–8. doi:10.1097/MAT.0b013e318199c167. PMID 19282746.
- ↑ "Inolivent". http://www.inolivent.ca/?lang=en.
- ↑ Kohlhauer, Matthias; Lidouren, Fanny; Remy-Jouet, Isabelle; Mongardon, Nicolas; Adam, Clovis; Bruneval, Patrick; Hocini, Hakim; Levy, Yves et al. (Oct 2015). "Hypothermic Total Liquid Ventilation Is Highly Protective Through Cerebral Hemodynamic Preservation and Sepsis-Like Mitigation After Asphyxial Cardiac Arrest*" (in en). Critical Care Medicine 43 (10): e420–e430. doi:10.1097/CCM.0000000000001160. ISSN 0090-3493. PMID 26110489. https://zenodo.org/record/895839.
- ↑ Clark, L. C.; Gollan, F. (1966-06-24). "Survival of Mammals Breathing Organic Liquids Equilibrated with Oxygen at Atmospheric Pressure" (in en). Science 152 (3730): 1755–1756. doi:10.1126/science.152.3730.1755. ISSN 0036-8075. PMID 5938414. Bibcode: 1966Sci...152.1755C.
- ↑ Hlastala, Michael P.; Souders, Jennifer E. (Jul 2001). "Perfluorocarbon Enhanced Gas Exchange: The Easy Way" (in en). American Journal of Respiratory and Critical Care Medicine 164 (1): 1–2. doi:10.1164/ajrccm.164.1.2104021a. ISSN 1073-449X. PMID 11435228. "A significant positive step was the use of PFC-associated gas exchange, now termed partial liquid ventilation (PLV).".
- ↑ Hirschl, Ronald B.; Pranikoff, T; Wise, C; Overbeck, MC et al. (1996-02-07). "Initial Experience With Partial Liquid Ventilation in Adult Patients With the Acute Respiratory Distress Syndrome" (in en). JAMA: The Journal of the American Medical Association 275 (5): 383–389. doi:10.1001/jama.1996.03530290053037. ISSN 0098-7484. PMID 8569018.
- ↑ Verbrugge, S.J.C.; Lachmann, B. (1997-09-01). "Partial liquid ventilation". European Respiratory Journal 10 (9): 1937–1939. doi:10.1183/09031936.97.10091937. PMID 9311481.[yes|permanent dead link|dead link}}] (editorial)
- ↑ Bleyl, Jorg U.; Ragaller, Maximilian; Tscho, Uwe; Regner, Mike; Kanzow, Maria; Hubler, Matthias; Rasche, Stefan; Albrecht, Michael (Aug 1999). "Vaporized Perfluorocarbon Improves Oxygenation and Pulmonary Function in an Ovine Model of Acute Respiratory Distress Syndrome" (in en). Anesthesiology 91 (2): 461–469. doi:10.1097/00000542-199908000-00021. ISSN 0003-3022. PMID 10443610. "Vaporization is a new application technique for perfluorocarbon that significantly improved oxygenation and pulmonary function in oleic acid-induced lung injury.".
- ↑ Kandler, Michael A.; von der HARDT, Katharina; Schoof, Ellen; Dötsch, Jörg; Rascher, Wolfgang (Jul 2001). "Persistent Improvement of Gas Exchange and Lung Mechanics by Aerosolized Perfluorocarbon" (in en). American Journal of Respiratory and Critical Care Medicine 164 (1): 31–35. doi:10.1164/ajrccm.164.1.2010049. ISSN 1073-449X. PMID 11435235. "Aerosolized perfluorocarbon improved pulmonary gas exchange and lung mechanics as effectively as PLV did in surfactant-depleted piglets, and the improvement was sustained longer.".
- ↑ Von Der Hardt, Katharina; Schoof, Ellen; Kandler, Michael A; Dötsch, Jörg; Rascher, Wolfgang (Feb 2002). "Aerosolized Perfluorocarbon Suppresses Early Pulmonary Inflammatory Response in a Surfactant-Depleted Piglet Model". Pediatric Research 51 (2): 177–182. doi:10.1203/00006450-200202000-00009. ISSN 0031-3998. PMID 11809911. "In a surfactant-depleted piglet model, aerosol therapy with perfluorocarbon but not LV-PLV reduces the initial pulmonary inflammatory reaction at least as potently as PLV at FRC volume.".
- ↑ Wolfson, Marla R.; Kechner, Nancy E.; Roache, Robert F.; Dechadarevian, Jean-Pierre et al. (Feb 1998). "Perfluorochemical rescue after surfactant treatment: effect of perflubron dose and ventilatory frequency" (in en). Journal of Applied Physiology 84 (2): 624–640. doi:10.1152/jappl.1998.84.2.624. ISSN 8750-7587. PMID 9475875.
- ↑ Stavis, Robert L; Wolfson, Marla R; Cox, Cynthia; Kechner, Nancy; Shaffer, Thomas H (Jan 1998). "Physiologic, Biochemical, and Histologic Correlates Associated with Tidal Liquid Ventilation". Pediatric Research 43 (1): 132–138. doi:10.1203/00006450-199801000-00020. ISSN 0031-3998. PMID 9432124.
- ↑ Wolfson, Marla R.; Shaffer, Thomas H. (Jun 2005). "Pulmonary applications of perfluorochemical liquids: Ventilation and beyond" (in en). Paediatric Respiratory Reviews 6 (2): 117–127. doi:10.1016/j.prrv.2005.03.010. PMID 15911457.
- ↑ Greenspan, JS; Wolfson, MR; Rubenstein, SD; Shaffer, TH (1989). "Liquid ventilation of preterm baby". The Lancet 2 (8671): 1095. doi:10.1016/S0140-6736(89)91101-X. PMID 2572810.
- ↑ Greenspan, Jay S.; Wolfson, Marla R.; Rubenstein, S. David; Shaffer, Thomas H. (Jul 1990). "Liquid ventilation of human preterm neonates" (in en). The Journal of Pediatrics 117 (1): 106–111. doi:10.1016/S0022-3476(05)82457-6. PMID 2115078.
- ↑ Leach, CL; Greenspan, JS; Rubenstein, SD; Shaffer, TH et al. (September 1996). "Partial liquid ventilation with perflubron in premature infants with severe respiratory distress syndrome. The LiquiVent Study Group". The New England Journal of Medicine 335 (11): 761–7. doi:10.1056/NEJM199609123351101. PMID 8778584.
- ↑ Greenspan, J. S.; Fox, W. W.; Rubenstein, S. D.; Wolfson, M. R.; Spinner, S. S.; Shaffer, T. H.; Philadelphia Liquid Ventilation Consortium (1997-01-01). "Partial Liquid Ventilation in Critically Ill Infants Receiving Extracorporeal Life Support" (in en). Pediatrics 99 (1): E2. doi:10.1542/peds.99.1.e2. ISSN 0031-4005. PMID 9096170.
- ↑ 30.0 30.1 30.2 Brunelli, Luca; Hamilton, Eric; Davis, Jonathan M; Koo, Hshi-Chi et al. (Jul 2006). "Perfluorochemical Liquids Enhance Delivery of Superoxide Dismutase to the Lungs of Juvenile Rabbits". Pediatric Research 60 (1): 65–70. doi:10.1203/01.pdr.0000219392.73509.70. ISSN 0031-3998. PMID 16690961.
- ↑ Nakstad, Britt; Wolfson, Marla R.; Shaffer, Thomas H.; Kähler, Hanne; Lindemann, Rolf; Fugelseth, Drude; Lyberg, Torstein (Sep 2001). "Perfluorochemical liquids modulate cell-mediated inflammatory responses" (in en). Critical Care Medicine 29 (9): 1731–1737. doi:10.1097/00003246-200109000-00013. ISSN 0090-3493. PMID 11546973.
- ↑ Ramesh Babu, Polani B.; Chidekel, Aaron; Shaffer, Thomas H. (Mar 2005). "Hyperoxia-induced changes in human airway epithelial cells: The protective effect of perflubron" (in en). Pediatric Critical Care Medicine 6 (2): 188–194. doi:10.1097/01.PCC.0000154944.67042.4F. ISSN 1529-7535. PMID 15730607.
- ↑ 33.0 33.1 Cox, Cynthia A.; Cullen, Aaron B.; Wolfson, Marla R.; Shaffer, Thomas H. (Aug 2001). "Intratracheal administration of perfluorochemical-gentamicin suspension: A comparison to intravenous administration in normal and injured lungs" (in en). Pediatric Pulmonology 32 (2): 142–151. doi:10.1002/ppul.1100. ISSN 8755-6863. PMID 11477731.
- ↑ 34.0 34.1 Fox, W. W.; Weis, C. M.; Cox, C.; Farina, C. et al. (1997-11-01). "Pulmonary Administration of Gentamicin During Liquid Ventilation in a Newborn Lamb Lung Injury Model" (in en). Pediatrics 100 (5): e5. doi:10.1542/peds.100.5.e5. ISSN 0031-4005. PMID 9346999.
- ↑ Wolfson, Marla R.; Greenspan, Jay S.; Shaffer, Thomas H. (1 April 1996). "Pulmonary Administration of Vasoactive Substances by Perfluorochemical Ventilation" (in en). Pediatrics 97 (4): 449–455. doi:10.1542/peds.97.4.449. ISSN 0031-4005. PMID 8632927. https://pediatrics.aappublications.org/content/97/4/449.
- ↑ Kimless-Garber, D.B.; Wolfson, M.R.; Carlsson, C.; Shaffer, T.H. (May 1997). "Halothane administration during liquid ventilation" (in en). Respiratory Medicine 91 (5): 255–262. doi:10.1016/S0954-6111(97)90028-7. PMID 9176643.
- ↑ Zelinka, M. A.; Wolfson, M. R.; Calligaro, I.; Rubenstein, S. D.; Greenspan, J. S.; Shaffer, T. H. (21 April 1997). "A comparison of intratracheal and intravenous administration of gentamicin during liquid ventilation". European Journal of Pediatrics 156 (5): 401–404. doi:10.1007/s004310050625. ISSN 0340-6199. PMID 9177987.
- ↑ Lisby, Dee Ann; Ballard, Philip L.; Fox, William W.; Wolfson, Marla R.; Shaffer, Thomas H.; Gonzales, Linda W. (20 May 1997). "Enhanced Distribution of Adenovirus-Mediated Gene Transfer to Lung Parenchyma by Perfluorochemical Liquid" (in en). Human Gene Therapy 8 (8): 919–928. doi:10.1089/hum.1997.8.8-919. ISSN 1043-0342. PMID 9195214.
- ↑ Cullen, A.B.; Cox, C.A.; Hipp, S.J.; Wolfson, M.R.; Shaffer, T.H. (Nov 1999). "Intra-tracheal delivery strategy of gentamicin with partial liquid ventilation" (in en). Respiratory Medicine 93 (11): 770–778. doi:10.1016/S0954-6111(99)90261-5. PMID 10603625.
- ↑ Chappell, S.E.; Wolfson, M.R.; Shaffer, T.H. (Jul 2001). "A comparison of surfactant delivery with conventional mechanical ventilation and partial liquid ventilation in meconium aspiration injury" (in en). Respiratory Medicine 95 (7): 612–617. doi:10.1053/rmed.2001.1114. PMID 11453320.
- ↑ Costantino, Maria-Laura; Shaffer, Thomas; Wauer, Roland R.; Rüdiger, Mario (July 2006). "The 5th European Symposium on Perfluorocarbon (PFC) Application" (in en). ASAIO Journal 52 (4): 483–484. doi:10.1097/00002480-200607000-00021. ISSN 1058-2916. PMID 16883132.
- ↑ Hirschl, R. B.; Pranikoff, T.; Wise, C.; Overbeck, M. C.; Gauger, P.; Schreiner, R. J.; Dechert, R.; Bartlett, R. H. (1996-02-07). "Initial experience with partial liquid ventilation in adult patients with the acute respiratory distress syndrome". JAMA 275 (5): 383–389. doi:10.1001/jama.1996.03530290053037. ISSN 0098-7484. PMID 8569018. https://pubmed.ncbi.nlm.nih.gov/8569018/.
- ↑ Darwin, MG (1996). "Liquid ventilation: A bypass on the way to bypass". BPI Tech Briefs 19. http://www.cryocare.org/index.cgi?subdir=bpi&url=tech19.txt.
- ↑ Harris, SB; Darwin, MG; Russell, SR; O'Farrell, JM et al. (2001). "Rapid (0.5°C/min) minimally invasive induction of hypothermia using cold perfluorochemical lung lavage in dogs". Resuscitation 50 (2): 189–204. doi:10.1016/S0300-9572(01)00333-1. PMID 11719148.
- ↑ Wolfson, Marla R.; Malone, Daniel J.; Wu, Jichuan; Hoffman, John; Rozenberg, Allan; Shaffer, Thomas H.; Barbut, Denise (Jun 2008). "Intranasal Perfluorochemical Spray for Preferential Brain Cooling in Sheep" (in en). Neurocritical Care 8 (3): 437–447. doi:10.1007/s12028-008-9064-0. ISSN 1541-6933. PMID 18266110.
- ↑ Castrén, Maaret; Nordberg, Per; Svensson, Leif; Taccone, Fabio; Vincent, Jean-Louise; Desruelles, Didier; Eichwede, Frank; Mols, Pierre et al. (17 Aug 2010). "Intra-Arrest Transnasal Evaporative Cooling: A Randomized, Prehospital, Multicenter Study (PRINCE: Pre-ROSC IntraNasal Cooling Effectiveness)" (in en). Circulation 122 (7): 729–736. doi:10.1161/CIRCULATIONAHA.109.931691. ISSN 0009-7322. PMID 20679548.
- ↑ Busch, H.-J.; Eichwede, F.; Födisch, M.; Taccone, F.S.; Wöbker, G.; Schwab, T.; Hopf, H.-B.; Tonner, P. et al. (Aug 2010). "Safety and feasibility of nasopharyngeal evaporative cooling in the emergency department setting in survivors of cardiac arrest" (in en). Resuscitation 81 (8): 943–949. doi:10.1016/j.resuscitation.2010.04.027. PMID 20627524.
- ↑ Lippsett, Lonny (5 April 2005). "Even Sperm Whales Get the Bends". Oceanus 44 (1). http://www.whoi.edu/oceanus/viewArticle.do?id=4720. Retrieved 3 August 2010.
- ↑ Kylstra, J. A. (Sep 1974). "Liquid breathing". Undersea Biomedical Research 1 (3): 259–269. ISSN 0093-5387. PMID 4619862.
- ↑ 50.0 50.1 Matthews, W. H.; Kylstra, J. A. (Jun 1976). "A fluorocarbon emulsion with a high solubility for CO2". Undersea Biomedical Research 3 (2): 113–120. ISSN 0093-5387. PMID 951821.
- ↑ Miyamoto, Yoshimi; Mikami, Tomohisa (1976). "Maximum Capacity Of Ventilation And Efficiency Of Gas Exchange During Liquid Breathing In Guinea Pigs" (in en). The Japanese Journal of Physiology 26 (6): 603–618. doi:10.2170/jjphysiol.26.603. ISSN 1881-1396. PMID 1030748.
- ↑ Sherwood, Lauralee; Klandorf, Hillar; Yancey, Paul H. (2005). Animal Physiology: From Genes to Organisms. Southbank, Victoria, Australia: Thomson/Brooks/Cole. ISBN 978-0-534-55404-0. OCLC 224468651.
- ↑ Koen, Peter A; Wolfson, Marla R; Shaffer, Thomas H (Sep 1988). "Fluorocarbon Ventilation: Maximal Expiratory Flows and CO2 Elimination". Pediatric Research 24 (3): 291–296. doi:10.1203/00006450-198809000-00003. ISSN 0031-3998. PMID 3145482.
- ↑ Matthews, W. H.; Balzer, R. H.; Shelburne, J. D.; Pratt, P. C.; Kylstra, J. A. (Dec 1978). "Steady-state gas exchange in normothermic, anesthetized, liquid-ventilated dogs". Undersea Biomedical Research 5 (4): 341–354. ISSN 0093-5387. PMID 153624.
- ↑ Taylor, Jerome (20 November 2010). "Into the abyss: The diving suit that turns men into fish". The Independent. Independent Print Ltd. https://www.independent.co.uk/news/science/into-the-abyss-the-diving-suit-that-turns-men-into-fish-2139167.html.
- ↑ Artificial gills for deep diving without incurring the bends and for scavenging O2 from and dispelling CO2 into water or thin air US Patent #8,631,788, published 21 Jan 2014.
- ↑ Hoepfner, Michael T.; Schultz, Marian C.; Schultz, James T. (Winter 2004). "Libelle Self-Contained Anti-G Ensemble: Overcoming Negative Transfer". The Journal of Aviation/Aerospace Education and Research (Daytona Beach, FL: Embry-Riddle Aeronautical University) 13 (2). OCLC 844961259. https://commons.erau.edu/cgi/viewcontent.cgi?article=1555&context=jaaer.
- ↑ Guyton, Arthur C. (1986). "Aviation, Space, and Deep Sea Diving Physiology". Textbook of Medical Physiology (7th ed.). W. B. Saunders Company. p. 533.
- ↑ Westerfeld, Scott (2003). The Risen Empire. Little, Brown Book. ISBN 978-0-7653-0555-8. https://books.google.com/books?id=XsXmQYq0LiMC&pg=PT23.
- ↑ McNeill, Graham (2008). Mechanicum: war comes to Mars. Horus Heresy. 9. Cover art & illustration by Neil Roberts; map by Adrian Wood (1st UK ed.). Nottingham, UK: Black Library. pp. 64, 149. ISBN 978-1-84416-664-0. The amniotic tanks are referenced in several other places in the novel.
- ↑ Cixin, Liu (2008). The Dark Forest. Head of Zeus. ISBN 978-1784971595.
- ↑ van Eekhout, Greg (2014). California Bones. Macmillan. ISBN 978-0765328557. https://books.google.com/books?id=bMxiAwAAQBAJ.
- ↑ Harmetz, Aljean (August 6, 1989). 'The Abyss': A Foray Into Deep Waters. https://www.nytimes.com/1989/08/06/movies/film-the-abyss-a-foray-into-deep-waters.html.
- ↑ "About Capsuleers - EVE Fiction - EVE Online Forums". https://forums.eveonline.com/default.aspx?g=posts&m=3479925#post3479925.
External links
- Here, Breathe This Liquid, from Discover Magazine
- Miracle Girl, from Reader's Digest
 |
 KSF
KSF