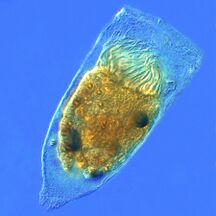
Marine microorganisms
Topic: Biology
 From HandWiki - Reading time: 75 min
From HandWiki - Reading time: 75 min
Marine microorganisms are defined by their habitat as microorganisms living in a marine environment, that is, in the saltwater of a sea or ocean or the brackish water of a coastal estuary. A microorganism (or microbe) is any microscopic living organism or virus, that is too small to see with the unaided human eye without magnification. Microorganisms are very diverse. They can be single-celled[1] or multicellular and include bacteria, archaea, viruses and most protozoa, as well as some fungi, algae, and animals, such as rotifers and copepods. Many macroscopic animals and plants have microscopic juvenile stages. Some microbiologists also classify viruses as microorganisms, but others consider these as non-living.[2][3]
Marine microorganisms have been variously estimated to make up about 70%,[4] or about 90%,[5][6] of the biomass in the ocean. Taken together they form the marine microbiome. Over billions of years this microbiome has evolved many life styles and adaptations and come to participate in the global cycling of almost all chemical elements.[7] Microorganisms are crucial to nutrient recycling in ecosystems as they act as decomposers. They are also responsible for nearly all photosynthesis that occurs in the ocean, as well as the cycling of carbon, nitrogen, phosphorus and other nutrients and trace elements.[8] Marine microorganisms sequester large amounts of carbon and produce much of the world's oxygen.
A small proportion of marine microorganisms are pathogenic, causing disease and even death in marine plants and animals.[9] However marine microorganisms recycle the major chemical elements, both producing and consuming about half of all organic matter generated on the planet every year. As inhabitants of the largest environment on Earth, microbial marine systems drive changes in every global system.
In July 2016, scientists reported identifying a set of 355 genes from the last universal common ancestor (LUCA) of all life on the planet, including the marine microorganisms.[10] Despite its diversity, microscopic life in the oceans is still poorly understood. For example, the role of viruses in marine ecosystems has barely been explored even in the beginning of the 21st century.[11]
| Part of a series of overviews on |
| Marine life |
|---|
Overview
1. What is out there? Which microorganisms are present in our seas and oceans and in what numbers
do they occur?
2. What are they doing? What functions do each of these microorganisms perform in the marine environment and how do they contribute to the global cycles of energy and matter?
– European Science Foundation, 2012[12]:14
Microorganisms make up about 70% of the marine biomass.[4] A microorganism, or microbe, is a microscopic organism too small to be recognised adequately with the naked eye. In practice, that includes organisms smaller than about 0.1 mm.[12]:13
Such organisms can be single-celled[1] or multicellular. Microorganisms are diverse and include all bacteria and archaea, most protists including algae, protozoa and fungal-like protists, as well as certain microscopic animals such as rotifers. Many macroscopic animals and plants have microscopic juvenile stages. Some microbiologists also classify viruses as microorganisms, but others consider these as non-living.[2][3]
Microorganisms are crucial to nutrient recycling in ecosystems as they act as decomposers. Some microorganisms are pathogenic, causing disease and even death in plants and animals.[9] As inhabitants of the largest environment on Earth, microbial marine systems drive changes in every global system. Microbes are responsible for virtually all the photosynthesis that occurs in the ocean, as well as the cycling of carbon, nitrogen, phosphorus and other nutrients and trace elements.[8]
| Marine microorganisms |
| ||||||||||||||||||||||||

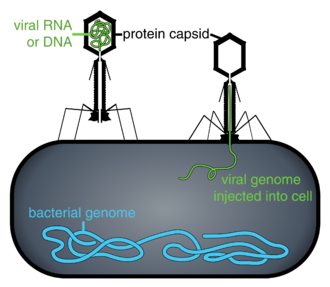
Microscopic life undersea is diverse and still poorly understood, such as for the role of viruses in marine ecosystems.[13] Most marine viruses are bacteriophages, which are harmless to plants and animals, but are essential to the regulation of saltwater and freshwater ecosystems.[14] They infect and destroy bacteria in aquatic microbial communities, and are the most important mechanism of recycling carbon in the marine environment. The organic molecules released from the dead bacterial cells stimulate fresh bacterial and algal growth.[15] Viral activity may also contribute to the biological pump, the process whereby carbon is sequestered in the deep ocean.[16]
A stream of airborne microorganisms circles the planet above weather systems but below commercial air lanes.[17] Some peripatetic microorganisms are swept up from terrestrial dust storms, but most originate from marine microorganisms in sea spray. In 2018, scientists reported that hundreds of millions of viruses and tens of millions of bacteria are deposited daily on every square meter around the planet.[18][19]
Microscopic organisms live throughout the biosphere. The mass of prokaryote microorganisms — which includes bacteria and archaea, but not the nucleated eukaryote microorganisms — may be as much as 0.8 trillion tons of carbon (of the total biosphere mass, estimated at between 1 and 4 trillion tons).[20] Single-celled barophilic marine microbes have been found at a depth of 10,900 m (35,800 ft) in the Mariana Trench, the deepest spot in the Earth's oceans.[21][22] Microorganisms live inside rocks 580 m (1,900 ft) below the sea floor under 2,590 m (8,500 ft) of ocean off the coast of the northwestern United States ,[21][23] as well as 2,400 m (7,900 ft; 1.5 mi) beneath the seabed off Japan.[24] The greatest known temperature at which microbial life can exist is 122 °C (252 °F) (Methanopyrus kandleri).[25] In 2014, scientists confirmed the existence of microorganisms living 800 m (2,600 ft) below the ice of Antarctica.[26][27] According to one researcher, "You can find microbes everywhere — they're extremely adaptable to conditions, and survive wherever they are."[21] Marine microorganisms serve as "the foundation of all marine food webs, recycling major elements and producing and consuming about half the organic matter generated on Earth each year".[28][29]
Marine viruses

including viral infection of bacteria, phytoplankton and fish[30]
A virus is a small infectious agent that replicates only inside the living cells of other organisms. Viruses can infect all types of life forms, from animals and plants to microorganisms, including bacteria and archaea.[31]
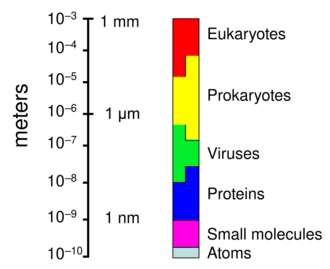
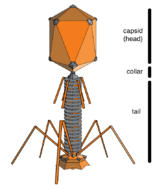
When not inside an infected cell or in the process of infecting a cell, viruses exist in the form of independent particles. These viral particles, also known as virions, consist of two or three parts: (i) the genetic material (genome) made from either DNA or RNA, long molecules that carry genetic information; (ii) a protein coat called the capsid, which surrounds and protects the genetic material; and in some cases (iii) an envelope of lipids that surrounds the protein coat when they are outside a cell. The shapes of these virus particles range from simple helical and icosahedral forms for some virus species to more complex structures for others. Most virus species have virions that are too small to be seen with an optical microscope. The average virion is about one one-hundredth the size of the average bacterium.
The origins of viruses in the evolutionary history of life are unclear: some may have evolved from plasmids—pieces of DNA that can move between cells—while others may have evolved from bacteria. In evolution, viruses are an important means of horizontal gene transfer, which increases genetic diversity.[32] Viruses are considered by some to be a life form, because they carry genetic material, reproduce, and evolve through natural selection. However, they lack key characteristics (such as cell structure) that are generally considered necessary to count as life. Because they possess some but not all such qualities, viruses have been described as "organisms at the edge of life"[33] and as replicators.[34]
Viruses are found wherever there is life and have probably existed since living cells first evolved.[35] The origin of viruses is unclear because they do not form fossils, so molecular techniques have been used to compare the DNA or RNA of viruses and are a useful means of investigating how they arose.[36]
Viruses are now recognised as ancient and as having origins that pre-date the divergence of life into the three domains.[37]
Opinions differ on whether viruses are a form of life or organic structures that interact with living organisms.[38] They are considered by some to be a life form, because they carry genetic material, reproduce by creating multiple copies of themselves through self-assembly, and evolve through natural selection. However they lack key characteristics such as a cellular structure generally considered necessary to count as life. Because they possess some but not all such qualities, viruses have been described as replicators[39] and as "organisms at the edge of life".[33]
Phages
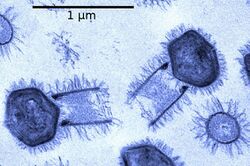
Bacteriophages, often just called phages, are viruses that parasite bacteria and archaea. Marine phages parasite marine bacteria and archaea, such as cyanobacteria.[40] They are a common and diverse group of viruses and are the most abundant biological entity in marine environments, because their hosts, bacteria, are typically the numerically dominant cellular life in the sea. Generally there are about 1 million to 10 million viruses in each mL of seawater, or about ten times more double-stranded DNA viruses than there are cellular organisms,[41][42] although estimates of viral abundance in seawater can vary over a wide range.[43][44] For a long time, tailed phages of the order Caudovirales seemed to dominate marine ecosystems in number and diversity of organisms.[40] However, as a result of more recent research, non-tailed viruses appear to be dominant in multiple depths and oceanic regions, followed by the Caudovirales families of myoviruses, podoviruses, and siphoviruses.[45] Phages belonging to the families Corticoviridae,[46] Inoviridae,[47] Microviridae,[48] and Autolykiviridae[49][50][51][52] are also known to infect diverse marine bacteria.
There are also archaean viruses which replicate within archaea: these are double-stranded DNA viruses with unusual and sometimes unique shapes.[53][54] These viruses have been studied in most detail in the thermophilic archaea, particularly the orders Sulfolobales and Thermoproteales.[55]
Role of viruses
Microorganisms make up about 70% of the marine biomass.[4] It is estimated viruses kill 20% of this biomass each day and that there are 15 times as many viruses in the oceans as there are bacteria and archaea. Viruses are the main agents responsible for the rapid destruction of harmful algal blooms,[56] which often kill other marine life.[57] The number of viruses in the oceans decreases further offshore and deeper into the water, where there are fewer host organisms.[16]
Viruses are an important natural means of transferring genes between different species, which increases genetic diversity and drives evolution.[32] It is thought that viruses played a central role in the early evolution, before the diversification of bacteria, archaea and eukaryotes, at the time of the last universal common ancestor of life on Earth.[58] Viruses are still one of the largest reservoirs of unexplored genetic diversity on Earth.[16]
Giant viruses

Viruses normally range in length from about 20 to 300 nanometers. This can be contrasted with the length of bacteria, which starts at about 400 nanometers. There are also giant viruses, often called giruses, typically about 1000 nanometers (one micron) in length. All giant viruses belongto phylum Nucleocytoviricota (NCLDV), together with poxviruses. The largest known of these is Tupanvirus. This genus of giant virus was discovered in 2018 in the deep ocean as well as a soda lake, and can reach up to 2.3 microns in total length.[59]
The discovery and subsequent characterization of giant viruses has triggered some debate concerning their evolutionary origins.[60] The two main hypotheses for their origin are that either they evolved from small viruses, picking up DNA from host organisms, or that they evolved from very complicated organisms into the current form which is not self-sufficient for reproduction.[61] What sort of complicated organism giant viruses might have diverged from is also a topic of debate. One proposal is that the origin point actually represents a fourth domain of life,[62][63] but this has been largely discounted.[64][65]
Prokaryotes
Marine bacteria
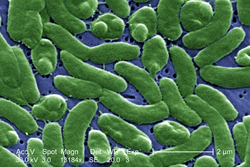

Bacteria constitute a large domain of prokaryotic microorganisms. Typically a few micrometres in length, bacteria have a number of shapes, ranging from spheres to rods and spirals. Bacteria were among the first life forms to appear on Earth, and are present in most of its habitats. Bacteria inhabit soil, water, acidic hot springs, radioactive waste,[66] and the deep portions of Earth's crust. Bacteria also live in symbiotic and parasitic relationships with plants and animals.
Once regarded as plants constituting the class Schizomycetes, bacteria are now classified as prokaryotes. Unlike cells of animals and other eukaryotes, bacterial cells do not contain a nucleus and rarely harbour membrane-bound organelles. Although the term bacteria traditionally included all prokaryotes, the scientific classification changed after the discovery in the 1990s that prokaryotes consist of two very different groups of organisms that evolved from an ancient common ancestor. These evolutionary domains are called Bacteria and Archaea.[67]
The ancestors of modern bacteria were unicellular microorganisms that were the first forms of life to appear on Earth, about 4 billion years ago. For about 3 billion years, most organisms were microscopic, and bacteria and archaea were the dominant forms of life.[68][69] Although bacterial fossils exist, such as stromatolites, their lack of distinctive morphology prevents them from being used to examine the history of bacterial evolution, or to date the time of origin of a particular bacterial species. However, gene sequences can be used to reconstruct the bacterial phylogeny, and these studies indicate that bacteria diverged first from the archaeal/eukaryotic lineage.[70] Bacteria were also involved in the second great evolutionary divergence, that of the archaea and eukaryotes. Here, eukaryotes resulted from the entering of ancient bacteria into endosymbiotic associations with the ancestors of eukaryotic cells, which were themselves possibly related to the Archaea.[71][72] This involved the engulfment by proto-eukaryotic cells of alphaproteobacterial symbionts to form either mitochondria or hydrogenosomes, which are still found in all known Eukarya. Later on, some eukaryotes that already contained mitochondria also engulfed cyanobacterial-like organisms. This led to the formation of chloroplasts in algae and plants. There are also some algae that originated from even later endosymbiotic events. Here, eukaryotes engulfed a eukaryotic algae that developed into a "second-generation" plastid.[73][74] This is known as secondary endosymbiosis.
The marine Thiomargarita namibiensis, largest known bacterium
Cyanobacteria blooms can contain lethal cyanotoxins
The chloroplasts of glaucophytes have a peptidoglycan layer, evidence suggesting their endosymbiotic origin from cyanobacteria.[75]
The bacterium Marinomonas arctica grows inside Arctic sea ice at subzero temperatures
Pelagibacter ubique and its relatives may be the most abundant organisms in the ocean, and it has been claimed that they are possibly the most abundant bacteria in the world. They make up about 25% of all microbial plankton cells, and in the summer they may account for approximately half the cells present in temperate ocean surface water. The total abundance of P. ubique and relatives is estimated to be about 2 × 1028 microbes.[76] However, it was reported in Nature in February 2013 that the bacteriophage HTVC010P, which attacks P. ubique, has been discovered and "it probably really is the commonest organism on the planet".[77][78]
The largest known bacterium, the marine Thiomargarita namibiensis, can be visible to the naked eye and sometimes attains 0.75 mm (750 μm).[79][80]
Marine archaea

The archaea (Greek for ancient[82]) constitute a domain and kingdom of single-celled microorganisms. These microbes are prokaryotes, meaning they have no cell nucleus or any other membrane-bound organelles in their cells.
Archaea were initially classified as bacteria, but this classification is outdated.[83] Archaeal cells have unique properties separating them from the other two domains of life, Bacteria and Eukaryota. The Archaea are further divided into multiple recognized phyla. Classification is difficult because the majority have not been isolated in the laboratory and have only been detected by analysis of their nucleic acids in samples from their environment.
Archaea and bacteria are generally similar in size and shape, although a few archaea have very strange shapes, such as the flat and square-shaped cells of Haloquadratum walsbyi.[84] Despite this morphological similarity to bacteria, archaea possess genes and several metabolic pathways that are more closely related to those of eukaryotes, notably the enzymes involved in transcription and translation. Other aspects of archaeal biochemistry are unique, such as their reliance on ether lipids in their cell membranes, such as archaeols. Archaea use more energy sources than eukaryotes: these range from organic compounds, such as sugars, to ammonia, metal ions or even hydrogen gas. Salt-tolerant archaea (the Haloarchaea) use sunlight as an energy source, and other species of archaea fix carbon; however, unlike plants and cyanobacteria, no known species of archaea does both. Archaea reproduce asexually by binary fission, fragmentation, or budding; unlike bacteria and eukaryotes, no known species forms spores.
Archaea are particularly numerous in the oceans, and the archaea in plankton may be one of the most abundant groups of organisms on the planet. Archaea are a major part of Earth's life and may play roles in both the carbon cycle and the nitrogen cycle. Thermoproteota (also known as eocytes or Crenarchaeota) are a phylum of archaea thought to be very abundant in marine environments and one of the main contributors to the fixation of carbon.[85]
Flat, square-shaped cells of the archaea Haloquadratum walsbyi
Methanosarcina barkeri, a marine archaea that produces methane
Thermophiles, such as Pyrolobus fumarii, survive well over 100 °C
Eukaryotes
All living organisms can be grouped as either prokaryotes or eukaryotes. Life originated as single-celled prokaryotes and later evolved into the more complex eukaryotes. In contrast to prokaryotic cells, eukaryotic cells are highly organised. Prokaryotes are the bacteria and archaea, while eukaryotes are the other life forms — protists, plants, fungi and animals. Protists are usually single-celled, while plants, fungi and animals are usually multi-celled.
It seems very plausible that the root of the eukaryotes lie within archaea; the closest relatives nowadays known may be the Heimdallarchaeota phylum of the proposed Asgard superphylum. This theory is a modern version of a scenario originally proposed in 1984 as Eocyte hypothesis, when Thermoproteota were the closest known archaeal relatives of eukaryotes then. A possible transitional form of microorganism between a prokaryote and a eukaryote was discovered in 2012 by Japanese scientists. Parakaryon myojinensis is a unique microorganism larger than a typical prokaryote, but with nuclear material enclosed in a membrane as in a eukaryote, and the presence of endosymbionts. This is seen to be the first plausible evolutionary form of microorganism, showing a stage of development from the prokaryote to the eukaryote.[86][87]
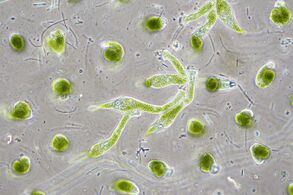
Marine protists
Protists are eukaryotes that cannot be classified as plants, fungi or animals. They are usually single-celled and microscopic. Life originated as single-celled prokaryotes (bacteria and archaea) and later evolved into more complex eukaryotes. Eukaryotes are the more developed life forms known as plants, animals, fungi and protists. The term protist came into use historically as a term of convenience for eukaryotes that cannot be strictly classified as plants, animals or fungi. They are not a part of modern cladistics, because they are paraphyletic (lacking a common ancestor).
By trophic mode
Protists can be broadly divided into four groups depending on whether their nutrition is plant-like, animal-like, fungal-like,[88] or a mixture of these.[89]
Protists according to how they get food
| |||||||
|---|---|---|---|---|---|---|---|
| Type of protist | Description | Example | Some other examples | ||||
| Plant-like | Algae
(see below) |
Autotrophic protists that make their own food without needing to consume other organisms, usually by using photosynthesis | 
|
Green algae, Pyramimonas | Red and brown algae, diatoms and some dinoflagellates. Plant-like protists are important components of phytoplankton discussed below. | ||
| Animal-like | Protozoans
|
Heterotrophic protists that get their food consuming other organisms (bacteria, archaea and small algae) | 
|
Radiolarian protist as drawn by Haeckel | Foraminiferans, and some marine amoebae, ciliates and flagellates. | ||
| Fungal-like | Slime moulds
and slime nets |
Saprotrophic protists that get their food from the remains of organisms that have broken down and decayed | 
|
Marine slime nets form labyrinthine networks of tubes in which amoeba without pseudopods can travel | Marine lichen | ||
| Mixotrops | Various
|
Mixotrophic and osmotrophic protists that get their food from a combination of the above | 
|
Euglena mutabilis, a photosynthetic flagellate | Many marine mixotrops are found among protists, particularly among ciliates and dinoflagellates[90] | ||
Protists are highly diverse organisms currently organised into 18 phyla, but are not easy to classify.[92][93] Studies have shown high protist diversity exists in oceans, deep sea-vents and river sediments, suggesting a large number of eukaryotic microbial communities have yet to be discovered.[94][95] There has been little research on mixotrophic protists, but recent studies in marine environments found mixotrophic protests contribute a significant part of the protist biomass.[90] Since protists are eukaryotes they possess within their cell at least one nucleus, as well as organelles such as mitochondria and Golgi bodies. Protists are asexual but can reproduce rapidly through mitosis or by fragmentation.
- Single-celled and microscopic protists
Fossil diatom frustule from 32 to 40 mya
Two dinoflagellates
Zooxanthellae is a photosynthetic algae that lives inside hosts like coral
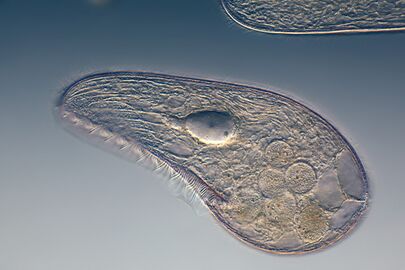
This ciliate is digesting cyanobacteria. The cytostome or mouth is at the bottom right.
In contrast to the cells of prokaryotes, the cells of eukaryotes are highly organised. Plants, animals and fungi are usually multi-celled and are typically macroscopic. Most protists are single-celled and microscopic. But there are exceptions. Some single-celled marine protists are macroscopic. Some marine slime molds have unique life cycles that involve switching between unicellular, colonial, and multicellular forms.[98] Other marine protist are neither single-celled nor microscopic, such as seaweed.
- Macroscopic protists (see also unicellular macroalgae →)
The single-celled giant amoeba has up to 1000 nuclei and reaches lengths of 5 mm
Gromia sphaerica is a large spherical testate amoeba which makes mud trails. Its diameter is up to 3.8 cm.[99]
Spiculosiphon oceana, a unicellular foraminiferan with an appearance and lifestyle that mimics a sponge, grows to 5 cm long.
The xenophyophore, another single-celled foraminiferan, lives in abyssal zones. It has a giant shell up to 20 cm across.[100]
Giant kelp, a brown algae, is not a true plant, yet it is multicellular and can grow to 50m
Protists have been described as a taxonomic grab bag of misfits where anything that doesn't fit into one of the main biological kingdoms can be placed.[101] Some modern authors prefer to exclude multicellular organisms from the traditional definition of a protist, restricting protists to unicellular organisms.[102][103] This more constrained definition excludes many brown, multicellular red and green algae, and slime molds.[104]
By locomotion
Another way of categorising protists is according to their mode of locomotion. Many unicellular protists, particularly protozoans, are motile and can generate movement using flagella, cilia or pseudopods. Cells which use flagella for movement are usually referred to as flagellates, cells which use cilia are usually referred to as ciliates, and cells which use pseudopods are usually referred to as amoeba or amoeboids. Other protists are not motile, and consequently have no movement mechanism.
Protists according to how they move
| ||||||||
|---|---|---|---|---|---|---|---|---|
| Type of protist | Movement mechanism | Description | Example | Other examples | ||||
| Motile | Flagellates | 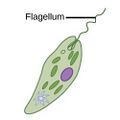
|
A flagellum (Latin for whip) is a lash-like appendage that protrudes from the cell body of some protists (as well as some bacteria). Flagellates use from one to several flagella for locomotion and sometimes as feeding and sensory organelle. | 
|
Cryptophytes | All dinoflagellates and nanoflagellates (choanoflagellates, silicoflagellates, most green algae)[105][106] (Other protists go through a phase as gametes when they have temporary flagellum – some radiolarians, foraminiferans and Apicomplexa)
| ||
| Ciliates | 
|
A cilium (Latin for eyelash) is a tiny flagellum. Ciliates use multiple cilia, which can number in many hundreds, to power themselves through the water. | 
|
Paramecium bursaria click to see cilia |
Foraminiferans, and some marine amoebae, ciliates and flagellates. | |||
| Amoebas (amoeboids) |
|
Amoeba have the ability to alter shape by extending and retracting pseudopods (Greek for false feet).[107] | Amoeba | Found in every major protist lineage. Amoeboid cells occur among the protozoans, but also in the algae and the fungi.[108][109] | ||||
| Not motile | none
|
Diatom | Diatoms, coccolithophores, and non‐motile species of Phaeocystis[106] Among protozoans the parasitic Apicomplexa are non‐motile. | |||||
Flagellates include bacteria as well as protists. The rotary motor model used by bacteria uses the protons of an electrochemical gradient in order to move their flagella. Torque in the flagella of bacteria is created by particles that conduct protons around the base of the flagellum. The direction of rotation of the flagella in bacteria comes from the occupancy of the proton channels along the perimeter of the flagellar motor.[110]
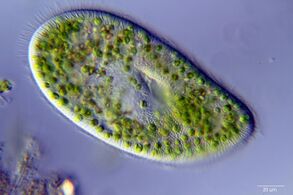
Ciliates generally have hundreds to thousands of cilia that are densely packed together in arrays. During movement, an individual cilium deforms using a high-friction power stroke followed by a low-friction recovery stroke. Since there are multiple cilia packed together on an individual organism, they display collective behavior in a metachronal rhythm. This means the deformation of one cilium is in phase with the deformation of its neighbor, causing deformation waves that propagate along the surface of the organism. These propagating waves of cilia are what allow the organism to use the cilia in a coordinated manner to move. A typical example of a ciliated microorganism is the Paramecium, a one-celled, ciliated protozoan covered by thousands of cilia. The cilia beating together allow the Paramecium to propel through the water at speeds of 500 micrometers per second.[111]
- Flagellate, ciliates and amoeba
Bacterial flagellum rotated by a molecular motor at its base
Paramecium feeding on bacteria
The ciliate Oxytricha trifallax with cilia clearly visible
Marine fungi
Over 1500 species of fungi are known from marine environments.[112] These are parasitic on marine algae or animals, or are saprobes feeding on dead organic matter from algae, corals, protozoan cysts, sea grasses, and other substrata.[113] Spores of many species have special appendages which facilitate attachment to the substratum.[114] Marine fungi can also be found in sea foam and around hydrothermal areas of the ocean.[115] A diverse range of unusual secondary metabolites is produced by marine fungi.[116]
Mycoplankton are saprotropic members of the plankton communities of marine and freshwater ecosystems.[117][118] They are composed of filamentous free-living fungi and yeasts associated with planktonic particles or phytoplankton.[119] Similar to bacterioplankton, these aquatic fungi play a significant role in heterotrophic mineralization and nutrient cycling.[120] While mostly microscopic, some mycoplankton can be up to 20 mm in diameter and over 50 mm in length.[121]
A typical milliliter of seawater contains about 103 to 104 fungal cells.[122] This number is greater in coastal ecosystems and estuaries due to nutritional runoff from terrestrial communities. A higher diversity of mycoplankton is found around coasts and in surface waters down to 1000 metres, with a vertical profile that depends on how abundant phytoplankton is.[123][124] This profile changes between seasons due to changes in nutrient availability.[125] Marine fungi survive in a constant oxygen deficient environment, and therefore depend on oxygen diffusion by turbulence and oxygen generated by photosynthetic organisms.[126]
Marine fungi can be classified as:[126]
- Lower fungi – adapted to marine habitats (zoosporic fungi, including mastigomycetes: oomycetes and chytridiomycetes)
- Higher fungi – filamentous, modified to planktonic lifestyle (hyphomycetes, ascomycetes, basidiomycetes). Most mycoplankton species are higher fungi.[123]
Lichens are mutualistic associations between a fungus, usually an ascomycete, and an alga or a cyanobacterium. Several lichens are found in marine environments.[127] Many more occur in the splash zone, where they occupy different vertical zones depending on how tolerant they are to submersion.[128] Some lichens live a long time; one species has been dated at 8,600 years.[129] However their lifespan is difficult to measure because what defines the same lichen is not precise.[130] Lichens grow by vegetatively breaking off a piece, which may or may not be defined as the same lichen, and two lichens of different ages can merge, raising the issue of whether it is the same lichen.[130]
The sea snail Littoraria irrorata damages plants of Spartina in the sea marshes where it lives, which enables spores of intertidal ascomycetous fungi to colonise the plant. The snail then eats the fungal growth in preference to the grass itself.[131]
According to fossil records, fungi date back to the late Proterozoic era 900-570 million years ago. Fossil marine lichens 600 million years old have been discovered in China.[132] It has been hypothesized that mycoplankton evolved from terrestrial fungi, likely in the Paleozoic era (390 million years ago).[133]
Marine microanimals
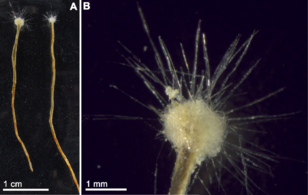
As juveniles, animals develop from microscopic stages, which can include spores, eggs and larvae. At least one microscopic animal group, the parasitic cnidarian Myxozoa, is unicellular in its adult form, and includes marine species. Other adult marine microanimals are multicellular. Microscopic adult arthropods are more commonly found inland in freshwater, but there are marine species as well. Microscopic adult marine crustaceans include some copepods, cladocera and tardigrades (water bears). Some marine nematodes and rotifers are also too small to be recognised with the naked eye, as are many loricifera, including the recently discovered anaerobic species that spend their lives in an anoxic environment.[134][135] Copepods contribute more to the secondary productivity and carbon sink of the world oceans than any other group of organisms.
- Marine microanimals
Over 10,000 marine species are copepods, small, often microscopic crustaceans
Darkfield photo of a gastrotrich, 0.06-3.0 mm long, a worm-like animal living between sediment particles
Armoured Pliciloricus enigmaticus, about 0.2 mm long, live in spaces between marine gravel
Rotifers, usually 0.1–0.5 mm long, may look like protists but are multicellular and belong to the Animalia
Tardigrades (water bears), about 0.5 mm long, are among the most resilient animals known
Primary producers
Primary producers are the autotroph organisms that make their own food instead of eating other organisms. This means primary producers become the starting point in the food chain for heterotroph organisms that do eat other organisms. Some marine primary producers are specialised bacteria and archaea which are chemotrophs, making their own food by gathering around hydrothermal vents and cold seeps and using chemosynthesis. However most marine primary production comes from organisms which use photosynthesis on the carbon dioxide dissolved in the water. This process uses energy from sunlight to convert water and carbon dioxide[136]:186–187 into sugars that can be used both as a source of chemical energy and of organic molecules that are used in the structural components of cells.[136]:1242 Marine primary producers are important because they underpin almost all marine animal life by generating most of the oxygen and food that provide other organisms with the chemical energy they need to exist.
The principal marine primary producers are cyanobacteria, algae and marine plants. The oxygen released as a by-product of photosynthesis is needed by nearly all living things to carry out cellular respiration. In addition, primary producers are influential in the global carbon and water cycles. They stabilize coastal areas and can provide habitats for marine animals. The term division has been traditionally used instead of phylum when discussing primary producers, but the International Code of Nomenclature for algae, fungi, and plants now accepts both terms as equivalents.[137]
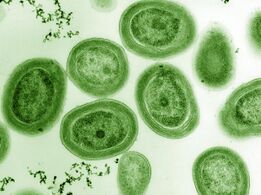
Cyanobacteria
Cyanobacteria were the first organisms to evolve an ability to turn sunlight into chemical energy. They form a phylum (division) of bacteria which range from unicellular to filamentous and include colonial species. They are found almost everywhere on earth: in damp soil, in both freshwater and marine environments, and even on Antarctic rocks.[139] In particular, some species occur as drifting cells floating in the ocean, and as such were amongst the first of the phytoplankton.
The first primary producers that used photosynthesis were oceanic cyanobacteria about 2.3 billion years ago.[140][141] The release of molecular oxygen by cyanobacteria as a by-product of photosynthesis induced global changes in the Earth's environment. Because oxygen was toxic to most life on Earth at the time, this led to the near-extinction of oxygen-intolerant organisms, a dramatic change which redirected the evolution of the major animal and plant species.[142]
The tiny (0.6 µm) marine cyanobacterium Prochlorococcus, discovered in 1986, forms today an important part of the base of the ocean food chain and accounts for much of the photosynthesis of the open ocean[143] and an estimated 20% of the oxygen in the Earth's atmosphere.[144] It is possibly the most plentiful genus on Earth: a single millilitre of surface seawater may contain 100,000 cells or more.[145]
Originally, biologists thought cyanobacteria was algae, and referred to it as "blue-green algae". The more recent view is that cyanobacteria are bacteria, and hence are not even in the same Kingdom as algae. Most authorities exclude all prokaryotes, and hence cyanobacteria from the definition of algae.[146][147]
Algae
Algae is an informal term for a widespread and diverse group of photosynthetic protists which are not necessarily closely related and are thus polyphyletic. Marine algae can be divided into six groups: green, red and brown algae, euglenophytes, dinoflagellates and diatoms.
Dinoflagellates and diatoms are important components of marine algae and have their own sections below. Euglenophytes are a phylum of unicellular flagellates with only a few marine members.
Not all algae are microscopic. Green, red and brown algae all have multicellular macroscopic forms that make up the familiar seaweeds. Green algae, an informal group, contains about 8,000 recognised species.[148] Many species live most of their lives as single cells or are filamentous, while others form colonies made up from long chains of cells, or are highly differentiated macroscopic seaweeds. Red algae, a (disputed) phylum contains about 7,000 recognised species,[149] mostly multicellular and including many notable seaweeds.[149][150] Brown algae form a class containing about 2,000 recognised species,[151] mostly multicellular and including many seaweeds such as kelp. Unlike higher plants, algae lack roots, stems, or leaves. They can be classified by size as microalgae or macroalgae.
Microalgae are the microscopic types of algae, not visible to the naked eye. They are mostly unicellular species which exist as individuals or in chains or groups, though some are multicellular. Microalgae are important components of the marine protists discussed above, as well as the phytoplankton discussed below. They are very diverse. It has been estimated there are 200,000-800,000 species of which about 50,000 species have been described.[152] Depending on the species, their sizes range from a few micrometers (µm) to a few hundred micrometers. They are specially adapted to an environment dominated by viscous forces.
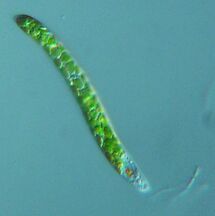
Chlorella vulgaris, a common green microalgae, in endosymbiosis with a ciliate[153]
Macroalgae are the larger, multicellular and more visible types of algae, commonly called seaweeds. Seaweeds usually grow in shallow coastal waters where they are anchored to the seafloor by a holdfast. Like microalgae, macroalgae (seaweeds) can be regarded as marine protists since they are not true plants. But they are not microorganisms, so they are not within the scope of this article.
Unicellular organisms are usually microscopic, less than one tenth of a millimeter long. There are exceptions. Mermaid's wineglass, a genus of subtropical green algae, is single-celled but remarkably large and complex in form with a single large nucleus, making it a model organism for studying cell biology.[154] Another single-celled algae, Caulerpa taxifolia, has the appearance of a vascular plant including "leaves" arranged neatly up stalks like a fern. Selective breeding in aquariums to produce hardier strains resulted in an accidental release into the Mediterranean where it has become an invasive species known colloquially as killer algae.[155]
Marine microplankton
Plankton (from Greek for wanderers) are a diverse group of organisms that live in the water column of large bodies of water but cannot swim against a current. As a result, they wander or drift with the currents.[156] Plankton are defined by their ecological niche, not by any phylogenetic or taxonomic classification. They are a crucial source of food for many marine animals, from forage fish to whales. Plankton can be divided into a plant-like component and an animal component.
Phytoplankton

Phytoplankton are the plant-like components of the plankton community ("phyto" comes from the Greek for plant). They are autotrophic (self-feeding), meaning they generate their own food and do not need to consume other organisms.
Phytoplankton perform three crucial functions: they generate nearly half of the world atmospheric oxygen, they regulate ocean and atmospheric carbon dioxide levels, and they form the base of the marine food web. When conditions are right, blooms of phytoplankton algae can occur in surface waters. Phytoplankton are r-strategists which grow rapidly and can double their population every day. The blooms can become toxic and deplete the water of oxygen. However, phytoplankton numbers are usually kept in check by the phytoplankton exhausting available nutrients and by grazing zooplankton.[159]
Phytoplankton consist mainly of microscopic photosynthetic eukaryotes which inhabit the upper sunlit layer in all oceans. They need sunlight so they can photosynthesize. Most phytoplankton are single-celled algae, but other phytoplankton are bacteria and some are protists.[160] Phytoplankton include cyanobacteria (above), diatoms, various other types of algae (red, green, brown, and yellow-green), dinoflagellates, euglenoids, coccolithophorids, cryptomonads, chlorophytes, prasinophytes, and silicoflagellates. They form the base of the primary production that drives the ocean food web, and account for half of the current global primary production, more than the terrestrial forests.[161]
- Phytoplankton
Colonial phytoplankton
The cyanobacterium Prochlorococcus accounts for much of the ocean's primary production
Green cyanobacteria scum washed up on a rock in California
Diatoms
Diatoms form a (disputed) phylum containing about 100,000 recognised species of mainly unicellular algae. Diatoms generate about 20 per cent of the oxygen produced on the planet each year,[96] take in over 6.7 billion metric tons of silicon each year from the waters in which they live,[162] and contribute nearly half of the organic material found in the oceans.
Diatoms are one of the most common types of phytoplankton
Diatoms are enclosed in protective silica (glass) shells called frustules. Each frustule is made from two interlocking parts covered with tiny holes through which the diatom exchanges nutrients and wastes.[159] The frustules of dead diatoms drift to the ocean floor where, over millions of years, they can build up as much as half a mile deep.[163]
Guinardia delicatula, a diatom responsible for algal blooms in the North Sea and the English Channel[164]
There are over 100,000 species of diatoms which account for 50% of the ocean's primary production

Coccolithophores
Coccolithophores are minute unicellular photosynthetic protists with two flagella for locomotion. Most of them are protected by a shell covered with ornate circular plates or scales called coccoliths. The coccoliths are made from calcium carbonate. The term coccolithophore derives from the Greek for a seed carrying stone, referring to their small size and the coccolith stones they carry. Under the right conditions they bloom, like other phytoplankton, and can turn the ocean milky white.[166]
The coccolithophore Emiliania huxleyi
Algae bloom of Emiliania huxleyi off the southern coast of England
Microbial rhodopsin
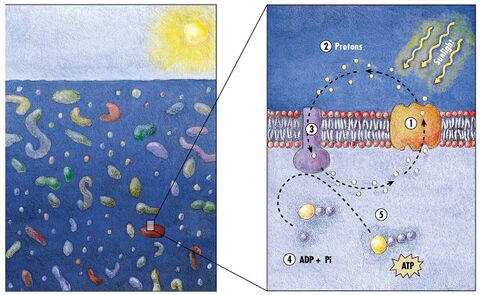
(2) it changes its configuration so a proton is expelled from the cell
(3) the chemical potential causes the proton to flow back to the cell
(4) thus generating energy
(5) in the form of adenosine triphosphate.[168]
Phototrophic metabolism relies on one of three energy-converting pigments: chlorophyll, bacteriochlorophyll, and retinal. Retinal is the chromophore found in rhodopsins. The significance of chlorophyll in converting light energy has been written about for decades, but phototrophy based on retinal pigments is just beginning to be studied.[169]
In 2000 a team of microbiologists led by Edward DeLong made a crucial discovery in the understanding of the marine carbon and energy cycles. They discovered a gene in several species of bacteria[171][172] responsible for production of the protein rhodopsin, previously unheard of in bacteria. These proteins found in the cell membranes are capable of converting light energy to biochemical energy due to a change in configuration of the rhodopsin molecule as sunlight strikes it, causing the pumping of a proton from inside out and a subsequent inflow that generates the energy.[173] The archaeal-like rhodopsins have subsequently been found among different taxa, protists as well as in bacteria and archaea, though they are rare in complex multicellular organisms.[174][175][176]
Research in 2019 shows these "sun-snatching bacteria" are more widespread than previously thought and could change how oceans are affected by global warming. "The findings break from the traditional interpretation of marine ecology found in textbooks, which states that nearly all sunlight in the ocean is captured by chlorophyll in algae. Instead, rhodopsin-equipped bacteria function like hybrid cars, powered by organic matter when available — as most bacteria are — and by sunlight when nutrients are scarce."[177][169]
There is an astrobiological conjecture called the Purple Earth hypothesis which surmises that original life forms on Earth were retinal-based rather than chlorophyll-based, which would have made the Earth appear purple instead of green.[178][179]
Redfield and f- ratios
During the 1930s Alfred C. Redfield found similarities between the composition of elements in phytoplankton and the major dissolved nutrients in the deep ocean.[180] Redfield proposed that the ratio of carbon to nitrogen to phosphorus (106:16:1) in the ocean was controlled by the phytoplankton's requirements, as phytoplankton subsequently release nitrogen and phosphorus as they remineralize. This ratio has become known as the Redfield ratio, and is used as a fundamental principle in describing the stoichiometry of seawater and phytoplankton evolution.[181]
However, the Redfield ratio is not a universal value and can change with things like geographical latitude.[182] Based on allocation of resources, phytoplankton can be classified into three different growth strategies: survivalist, bloomer and generalist. Survivalist phytoplankton has a high N:P ratio (>30) and contains an abundance of resource-acquisition machinery to sustain growth under scarce resources. Bloomer phytoplankton has a low N:P ratio (<10), contains a high proportion of growth machinery and is adapted to exponential growth. Generalist phytoplankton has similar N:P to the Redfield ratio and contain relatively equal resource-acquisition and growth machinery.[181]
The f-ratio is the fraction of total primary production fuelled by nitrate (as opposed to that fuelled by other nitrogen compounds such as ammonium). The ratio was originally defined by Richard Eppley and Bruce Peterson in one of the first papers estimating global oceanic production.[183]
Zooplankton
Zooplankton are the animal component of the planktonic community ("zoo" comes from the Greek for animal). They are heterotrophic (other-feeding), meaning they cannot produce their own food and must consume instead other plants or animals as food. In particular, this means they eat phytoplankton.
Zooplankton are generally larger than phytoplankton, mostly still microscopic but some can be seen with the naked eye. Many protozoans (single-celled protists that prey on other microscopic life) are zooplankton, including zooflagellates, foraminiferans, radiolarians, some dinoflagellates and marine microanimals. Macroscopic zooplankton (not generally covered in this article) include pelagic cnidarians, ctenophores, molluscs, arthropods and tunicates, as well as planktonic arrow worms and bristle worms.
Microzooplankton: major grazers of the plankton...
Many species of protozoa (eukaryotes) and bacteria (prokaryotes) prey on other microorganisms; the feeding mode is evidently ancient, and evolved many times in both groups.[184][185][186] Among freshwater and marine zooplankton, whether single-celled or multi-cellular, predatory grazing on phytoplankton and smaller zooplankton is common, and found in many species of nanoflagellates, dinoflagellates, ciliates, rotifers, a diverse range of meroplankton animal larvae, and two groups of crustaceans, namely copepods and cladocerans.[187]
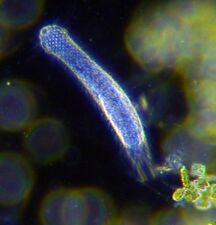
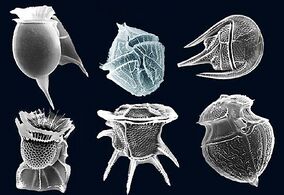
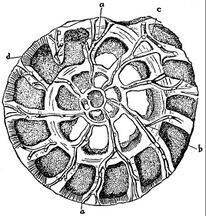
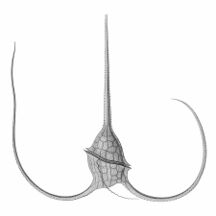
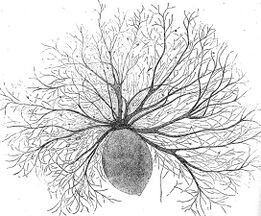
Radiolarians
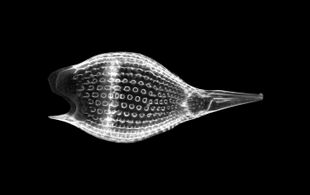
Radiolarians are unicellular predatory protists encased in elaborate globular shells usually made of silica and pierced with holes. Their name comes from the Latin for "radius". They catch prey by extending parts of their body through the holes. As with the silica frustules of diatoms, radiolarian shells can sink to the ocean floor when radiolarians die and become preserved as part of the ocean sediment. These remains, as microfossils, provide valuable information about past oceanic conditions.[188]
However acantharian radiolarians have shells made from strontium sulfate crystals
closely replicate some radiolarian shell patterns[189]
Foraminiferans
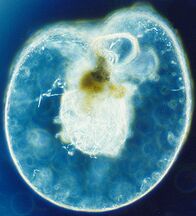
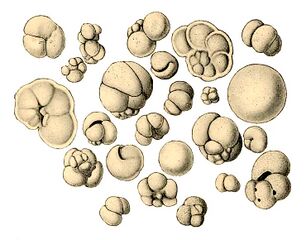
Like radiolarians, foraminiferans (forams for short) are single-celled predatory protists, also protected with shells that have holes in them. Their name comes from the Latin for "hole bearers". Their shells, often called tests, are chambered (forams add more chambers as they grow). The shells are usually made of calcite, but are sometimes made of agglutinated sediment particles or chiton, and (rarely) of silica. Most forams are benthic, but about 40 species are planktic.[190] They are widely researched with well established fossil records which allow scientists to infer a lot about past environments and climates.[188]
Live Ammonia tepida streaming granular ectoplasm for catching food
Fossil nummulitid forams of various sizes from the Eocene
The Egyptian pyramids were constructed from limestone that contained nummulites.[191]
A number of forams are mixotrophic (see below). These have unicellular algae as endosymbionts, from diverse lineages such as the green algae, red algae, golden algae, diatoms, and dinoflagellates.[190] Mixotrophic foraminifers are particularly common in nutrient-poor oceanic waters.[192] Some forams are kleptoplastic, retaining chloroplasts from ingested algae to conduct photosynthesis.[193]
Amoeba
Shell or test of a testate amoeba, Arcella sp.
Xenogenic testate amoeba covered in diatoms (from Penard's Amoeba Collection)
File:Amoeba engulfing diatom.ogv
Ciliates
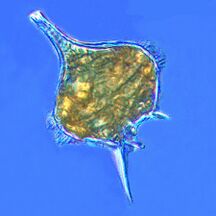
Mesodinium rubrum produce deep red blooms using enslaved chloroplasts from their algal prey [194]
Blepharisma americanum swimming in a drop of pond water with other microorganisms
Mixotrophs
A mixotroph is an organism that can use a mix of different sources of energy and carbon, instead of having a single trophic mode on the continuum from complete autotrophy at one end to heterotrophy at the other. It is estimated that mixotrophs comprise more than half of all microscopic plankton.[195] There are two types of eukaryotic mixotrophs: those with their own chloroplasts, and those with endosymbionts—and others that acquire them through kleptoplasty or by enslaving the entire phototrophic cell.[196]
The distinction between plants and animals often breaks down in very small organisms. Possible combinations are photo- and chemotrophy, litho- and organotrophy, auto- and heterotrophy or other combinations of these. Mixotrophs can be either eukaryotic or prokaryotic.[197] They can take advantage of different environmental conditions.[198]
Recent studies of marine microzooplankton found 30–45% of the ciliate abundance was mixotrophic, and up to 65% of the amoeboid, foram and radiolarian biomass was mixotrophic.[90]
Phaeocystis is an important algal genus found as part of the marine phytoplankton around the world. It has a polymorphic life cycle, ranging from free-living cells to large colonies.[199] It has the ability to form floating colonies, where hundreds of cells are embedded in a gel matrix, which can increase massively in size during blooms.[200] As a result, Phaeocystis is an important contributor to the marine carbon[201] and sulfur cycles.[202] Phaeocystis species are endosymbionts to acantharian radiolarians.[203][204]
Mixotrophic plankton that combine phototrophy and heterotrophy – table based on Stoecker et al., 2017[205]
| |||||||
|---|---|---|---|---|---|---|---|
| General types | Description | Example | Further examples | ||||
| Bacterioplankton | Photoheterotrophic bacterioplankton | 
|
Vibrio cholerae | Roseobacter spp. Erythrobacter spp. Gammaproteobacterial clade OM60 Widespread among bacteria and archaea | |||
| Phytoplankton | Called constitutive mixotrophs by Mitra et al., 2016.[206] Phytoplankton that eat: photosynthetic protists with inherited plastids and the capacity to ingest prey. | 
|
Ochromonas species | Ochromonas spp. Prymnesium parvum Dinoflagellate examples: Fragilidium subglobosum,Heterocapsa triquetra,Karlodinium veneficum,Neoceratium furca,Prorocentrum minimum | |||
| Zooplankton | Called nonconstitutive mixotrophs by Mitra et al., 2016.[206] Zooplankton that are photosynthetic: microzooplankton or metazoan zooplankton that acquire phototrophy through chloroplast retentiona or maintenance of algal endosymbionts. | ||||||
| Generalists | Protists that retain chloroplasts and rarely other organelles from many algal taxa | 
|
Most oligotrich ciliates that retain plastidsa | ||||
| Specialists | 1. Protists that retain chloroplasts and sometimes other organelles from one algal species or very closely related algal species | 
|
Dinophysis acuminata | Dinophysis spp. Myrionecta rubra | |||
| 2. Protists or zooplankton with algal endosymbionts of only one algal species or very closely related algal species | 
|
Noctiluca scintillans | Metazooplankton with algal endosymbionts Most mixotrophic Rhizaria (Acantharea, Polycystinea, and Foraminifera) Green Noctiluca scintillans | ||||
| aChloroplast (or plastid) retention = sequestration = enslavement. Some plastid-retaining species also retain other organelles and prey cytoplasm. | |||||||
- Mixoplankton
Tintinnid ciliate Favella
Euglena mutabilis, a photosynthetic flagellate
Dinoflagellates
Dinoflagellates are part of the algae group, and form a phylum of unicellular flagellates with about 2,000 marine species.[207] The name comes from the Greek "dinos" meaning whirling and the Latin "flagellum" meaning a whip or lash. This refers to the two whip-like attachments (flagella) used for forward movement. Most dinoflagellates are protected with red-brown, cellulose armour. Like other phytoplankton, dinoflagellates are r-strategists which under right conditions can bloom and create red tides. Excavates may be the most basal flagellate lineage.[105]
By trophic orientation dinoflagellates cannot be uniformly categorized. Some dinoflagellates are known to be photosynthetic, but a large fraction of these are in fact mixotrophic, combining photosynthesis with ingestion of prey (phagotrophy).[208] Some species are endosymbionts of marine animals and other protists, and play an important part in the biology of coral reefs. Others predate other protozoa, and a few forms are parasitic. Many dinoflagellates are mixotrophic and could also be classified as phytoplankton.
The toxic dinoflagellate Dinophysis acuta acquire chloroplasts from its prey. "It cannot catch the cryptophytes by itself, and instead relies on ingesting ciliates such as the red Myrionecta rubra, which sequester their chloroplasts from a specific cryptophyte clade (Geminigera/Plagioselmis/Teleaulax)".[205]
Gyrodinium, one of the few naked dinoflagellates which lack armour
Dinoflagellates often live in symbiosis with other organisms. Many nassellarian radiolarians house dinoflagellate symbionts within their tests.[210] The nassellarian provides ammonium and carbon dioxide for the dinoflagellate, while the dinoflagellate provides the nassellarian with a mucous membrane useful for hunting and protection against harmful invaders.[211] There is evidence from DNA analysis that dinoflagellate symbiosis with radiolarians evolved independently from other dinoflagellate symbioses, such as with foraminifera.[212]
Some dinoflagellates are bioluminescent. At night, ocean water can light up internally and sparkle with blue light because of these dinoflagellates.[213][214] Bioluminescent dinoflagellates possess scintillons, individual cytoplasmic bodies which contain dinoflagellate luciferase, the main enzyme involved in the luminescence. The luminescence, sometimes called the phosphorescence of the sea, occurs as brief (0.1 sec) blue flashes or sparks when individual scintillons are stimulated, usually by mechanical disturbances from, for example, a boat or a swimmer or surf.[215]
Tripos muelleri is recognisable by its U-shaped horns
Karenia brevis produces red tides highly toxic to humans[217]
Noctiluca scintillans, a bioluminescent dinoflagellate[218]
Marine sediments and microfossils
Sediments at the bottom of the ocean have two main origins, terrigenous and biogenous.
Terrigenous sediments account for about 45% of the total marine sediment, and originate in the erosion of rocks on land, transported by rivers and land runoff, windborne dust, volcanoes, or grinding by glaciers.
Biogenous sediments account for the other 55% of the total sediment, and originate in the skeletal remains of marine protists (single-celled plankton and benthos microorganisms). Much smaller amounts of precipitated minerals and meteoric dust can also be present. Ooze, in the context of a marine sediment, does not refer to the consistency of the sediment but to its biological origin. The term ooze was originally used by John Murray, the "father of modern oceanography", who proposed the term radiolarian ooze for the silica deposits of radiolarian shells brought to the surface during the Challenger expedition.[219] A biogenic ooze is a pelagic sediment containing at least 30 per cent from the skeletal remains of marine organisms.
Main types of biogenic ooze
| ||||||||
|---|---|---|---|---|---|---|---|---|
| type | mineral forms |
protist involved |
name of skeleton | typical size (mm) |
||||
| Siliceous ooze | SiO2 silica quartz glass opal chert |
diatom | 
|
frustule | 0.002 to 0.2[220] | 
|
diatom microfossil from 40 million years ago | |
| radiolarian | 
|
test or shell | 0.1 to 0.2 | 
|
elaborate silica shell of a radiolarian | |||
| Calcareous ooze | CaCO3 calcite aragonite limestone marble chalk |
foraminiferan | 
|
test or shell | under 1 | 
|
Calcified test of a planktic foraminiferan. There are about 10,000 living species of foraminiferans[221] | |
| coccolithophore | 
|
coccoliths | under 0.1[222] | 
|
Coccolithophores are the largest global source of biogenic calcium carbonate, and significantly contribute to the global carbon cycle.[223] They are the main constituent of chalk deposits such as the white cliffs of Dover. | |||
Diatomaceous earth is a soft, siliceous, sedimentary rock made up of microfossils in the form of the frustules (shells) of single cell diatoms (click to magnify)
Illustration of a Globigerina ooze
Shells (tests), usually made of calcium carbonate, from a foraminiferal ooze on the deep ocean floor

Opal can contain protist microfossils of diatoms, radiolarians, silicoflagellates and ebridians[225]


Within each colored area, the type of material shown is what dominates, although other materials are also likely to be present.
For further information, see here
Marine microbenthos
Marine microbenthos are microorganisms that live in the benthic zone of the ocean – that live near or on the seafloor, or within or on surface seafloor sediments. The word benthos comes from Greek, meaning "depth of the sea". Microbenthos are found everywhere on or about the seafloor of continental shelves, as well as in deeper waters, with greater diversity in or on seafloor sediments. In shallow waters, seagrass meadows, coral reefs and kelp forests provide particularly rich habitats. In photic zones benthic diatoms dominate as photosynthetic organisms. In intertidal zones changing tides strongly control opportunities for microbenthos.
Elphidium a widespread abundant genus of benthic forams
Heterohelix, an extinct genus of benthic forams
Both foraminifera and diatoms have planktonic and benthic forms, that is, they can drift in the water column or live on sediment at the bottom of the ocean. Either way, their shells end up on the seafloor after they die. These shells are widely used as climate proxies. The chemical composition of the shells are a consequence of the chemical composition of the ocean at the time the shells were formed. Past water temperatures can be also be inferred from the ratios of stable oxygen isotopes in the shells, since lighter isotopes evaporate more readily in warmer water leaving the heavier isotopes in the shells. Information about past climates can be inferred further from the abundance of forams and diatoms, since they tend to be more abundant in warm water.[226]
The sudden extinction event which killed the dinosaurs 66 million years ago also rendered extinct three-quarters of all other animal and plant species. However, deep-sea benthic forams flourished in the aftermath. In 2020 it was reported that researchers have examined the chemical composition of thousands of samples of these benthic forams and used their findings to build the most detailed climate record of Earth ever.[227][228]
Some endoliths have extremely long lives. In 2013 researchers reported evidence of endoliths in the ocean floor, perhaps millions of years old, with a generation time of 10,000 years.[229] These are slowly metabolizing and not in a dormant state. Some Actinomycetota found in Siberia are estimated to be half a million years old.[230][231][232]
Marine microbiomes
Symbiosis and holobionts

The concept of the holobiont was initially defined by Dr. Lynn Margulis in her 1991 book Symbiosis as a Source of Evolutionary Innovation as an assemblage of a host and the many other species living in or around it, which together form a discrete ecological unit.[234] The components of a holobiont are individual species or bionts, while the combined genome of all bionts is the hologenome.[235]
The concept has subsequently evolved since this original definition,[236] with the focus moving to the microbial species associated with the host. Thus the holobiont includes the host, virome, microbiome, and other members, all of which contribute in some way to the function of the whole.[237][238] A holobiont typically includes a eukaryote host and all of the symbiotic viruses, bacteria, fungi, etc. that live on or inside it.[239]
However, there is controversy over whether holobionts can be viewed as single evolutionary units.[240]
Sponge holobiont[242]
Seagrass holobiont[243]
Reef-building corals are well-studied holobionts that include the coral itself (a eukaryotic invertebrate within class Anthozoa), photosynthetic dinoflagellates called zooxanthellae (Symbiodinium), and associated bacteria and viruses.[245] Co-evolutionary patterns exist for coral microbial communities and coral phylogeny.[246]
Marine food web
Marine microorganisms play central roles in the marine food web.
The viral shunt pathway is a mechanism that prevents marine microbial particulate organic matter (POM) from migrating up trophic levels by recycling them into dissolved organic matter (DOM), which can be readily taken up by microorganisms.[247] Viral shunting helps maintain diversity within the microbial ecosystem by preventing a single species of marine microbe from dominating the micro-environment.[248] The DOM recycled by the viral shunt pathway is comparable to the amount generated by the other main sources of marine DOM.[249]
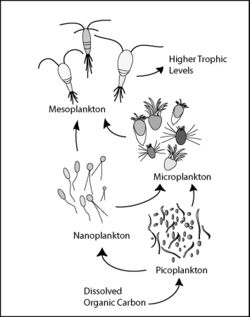

Pelagibacter ubique, the most abundant bacteria in the ocean, plays a major role in the global carbon cycle.
- Marine snow is the shower of organic particles that falls from upper waters to the deep ocean[251] It is a major exporter of carbon.
Niche communities
Sea ice microbial communities (SIMCO) refer to groups of microorganisms living within and at the interfaces of sea ice at the poles. The ice matrix they inhabit has strong vertical gradients of salinity, light, temperature and nutrients. Sea ice chemistry is most influenced by the salinity of the brine which affects the pH and the concentration of dissolved nutrients and gases. The brine formed during the melting sea ice creates pores and channels in the sea ice in which these microbes can live. As a result of these gradients and dynamic conditions, a higher abundance of microbes are found in the lower layer of the ice, although some are found in the middle and upper layers.[254]
Hydrothermal vents are located where the tectonic plates are moving apart and spreading. This allows water from the ocean to enter into the crust of the earth where it is heated by the magma. The increasing pressure and temperature forces the water back out of these openings, on the way out, the water accumulates dissolved minerals and chemicals from the rocks that it encounters. Vents can be characterized by temperature and chemical composition as diffuse vents which release clear relatively cool water usually below 30 °C, as white smokers which emit milky coloured water at warmer temperatures, about 200-330 °C, and as black smokers which emit water darkened by accumulated precipitates of sulfide at hot temperatures, about 300-400 °C.[255]
Hydrothermal vent microbial communities are microscopic unicellular organisms that live and reproduce in the chemically distinct area around hydrothermal vents. These include organisms in microbial mats, free floating cells, and bacteria in endosymbiotic relationships with animals. Because there is no sunlight at these depths, energy is provided by chemosynthesis where symbiotic bacteria and archaea form the bottom of the food chain and are able to support a variety of organisms such as giant tube worms and Pompeii worms. These organisms utilize this symbiotic relationship in order to utilize and obtain the chemical energy that is released at these hydrothermal vent areas.[256] Chemolithoautotrophic bacteria can derive nutrients and energy from the geological activity at a hydrothermal vent to fix carbon into organic forms.[257]
Viruses are also a part of the hydrothermal vent microbial community and their influence on the microbial ecology in these ecosystems is a burgeoning field of research.[258] Viruses are the most abundant life in the ocean, harboring the greatest reservoir of genetic diversity.[259] As their infections are often fatal, they constitute a significant source of mortality and thus have widespread influence on biological oceanographic processes, evolution and biogeochemical cycling within the ocean.[260] Evidence has been found however to indicate that viruses found in vent habitats have adopted a more mutualistic than parasitic evolutionary strategy in order to survive the extreme and volatile environment they exist in.[261] Deep-sea hydrothermal vents were found to have high numbers of viruses indicating high viral production.[262] Like in other marine environments, deep-sea hydrothermal viruses affect abundance and diversity of prokaryotes and therefore impact microbial biogeochemical cycling by lysing their hosts to replicate.[263] However, in contrast to their role as a source of mortality and population control, viruses have also been postulated to enhance survival of prokaryotes in extreme environments, acting as reservoirs of genetic information. The interactions of the virosphere with microorganisms under environmental stresses is therefore thought to aide microorganism survival through dispersal of host genes through horizontal gene transfer.[264]
Deep biosphere and dark matter

The deep biosphere is that part of the biosphere that resides below the first few meters of the surface. It extends at least 5 kilometers below the continental surface and 10.5 kilometers below the sea surface, with temperatures that may exceed 100 °C.
Above the surface living organisms consume organic matter and oxygen. Lower down, these are not available, so they make use of "edibles" (electron donors) such as hydrogen released from rocks by various chemical processes, methane, reduced sulfur compounds and ammonium. They "breathe" electron acceptors such as nitrates and nitrites, manganese and iron oxides, oxidized sulfur compounds and carbon dioxide.
There is very little energy at greater depths, and metabolism can be up to a million times slower than at the surface. Cells may live for thousands of years before dividing and there is no known limit to their age. The subsurface accounts for about 90% of the biomass in bacteria and archaea, and 15% of the total biomass for the biosphere. Eukaryotes are also found, mostly microscopic, but including some multicellular life. Viruses are also present and infect the microbes.
In 2018, researchers from the Deep Carbon Observatory announced that life forms, including 70% of the bacteria and archaea on Earth, totaling a biomass of 23 billion tonnes carbon, live up to 4.8 km (3.0 mi) deep underground, including 2.5 km (1.6 mi) below the seabed.[265][266][267] In 2019 microbial organisms were discovered living 7,900 feet (2,400 m) below the surface, breathing sulfur and eating rocks such as pyrite as their regular food source.[268][269][270] This discovery occurred in the oldest known water on Earth.[271]
In 2020 researchers reported they had found what could be the longest-living life forms ever: aerobic microorganisms which had been in quasi-suspended animation for up to 101.5 million years. The microorganisms were found in organically poor sediments 68.9 metres (226 feet) below the seafloor in the South Pacific Gyre (SPG), "the deadest spot in the ocean".[272][273]
To date biologists have been unable to culture in the laboratory the vast majority of microorganisms. This applies particularly to bacteria and archaea, and is due to a lack of knowledge or ability to supply the required growth conditions.[274][275] The term microbial dark matter has come to be used to describe microorganisms scientists know are there but have been unable to culture, and whose properties therefore remain elusive.[274] Microbial dark matter is unrelated to the dark matter of physics and cosmology, but is so-called for the difficulty in effectively studying it. It is hard to estimate its relative magnitude, but the accepted gross estimate is that less than one per cent of microbial species in a given ecological niche is culturable. In recent years effort is being put to decipher more of the microbial dark matter by means of learning their genome DNA sequence from environmental samples[276] and then by gaining insights to their metabolism from their sequenced genome, promoting the knowledge required for their cultivation.
Microbial diversity

Sampling techniques

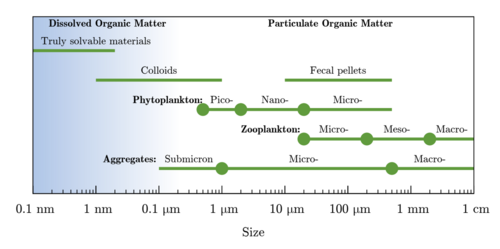
The blue background indicates the filtered volume required to obtain sufficient organism numbers for analysis.
Actual volumes from which organisms are sampled are always recorded.[280]
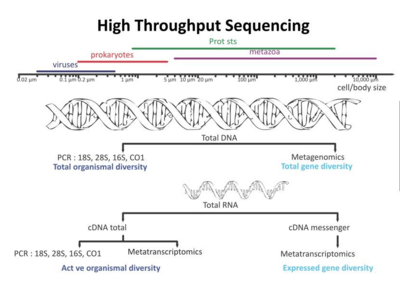
Identifying microorganisms
using modern imaging techniques.[280]
Traditionally, the phylogeny of microorganisms was inferred and their taxonomy was established based on studies of morphology. However, developments in molecular phylogenetics have allowed evolutionary relationship of species to be established by analyzing deeper characteristics, such as their DNA and protein sequences, for example ribosomal DNA.[281] The lack of easily accessible morphological features, such as those present in animals and plants, particularly hampered early efforts at classifying bacteria and archaea. This resulted in erroneous, distorted and confused classification, an example of which, noted Carl Woese, is Pseudomonas whose etymology ironically matched its taxonomy, namely "false unit".[282] Many bacterial taxa have been reclassified or redefined using molecular phylogenetics.

Methods of identifying microorganisms[285]
| |||
|---|---|---|---|
| Chromogenic media |
Microscopy techniques |
Biochemical techniques |
Molecular techniques |
Traditional media
|
Bright field Dark field SEM TEM CLSM ATM Inverted microscopy |
Spectrometry – FTIR – Raman spectrometry Mass spectrometry – GC – LC – MALDI-TOF – ESI Electrokinetic separation Microfluidic chip Propriety methods – Wickerham card – API – BBL Crystal – Vitek – Biolog |
PCR Real-time qPCR Rapid PCR PCR sequencing RFLP PFGE Ribotyping WGS MALDI-TOF MS |
Recent developments in molecular sequencing have allowed for the recovery of genomes in situ, directly from environmental samples and avoiding the need for culturing. This has led for example, to a rapid expansion in knowledge of the diversity of bacterial phyla. These techniques are genome-resolved metagenomics and single-cell genomics.
The new sequencing technologies and the accumulation of sequence data have resulted in a paradigm shift, highlighted both the ubiquity of microbial communities in association within higher organisms and the critical roles of microbes in ecosystem health.[286] These new possibilities have revolutionized microbial ecology, because the analysis of genomes and metagenomes in a high-throughput manner provides efficient methods for addressing the functional potential of individual microorganisms as well as of whole communities in their natural habitats.[287][288][289]
Using omics data

Omics is a term used informally to refer to branches of biology whose names end in the suffix -omics, such as genomics, proteomics, metabolomics, and glycomics. Marine Omics has recently emerged as a field of research of its own.[291] Omics aims at collectively characterising and quantifying pools of biological molecules that translate into the structure, function, and dynamics of an organism or organisms. For example, functional genomics aims at identifying the functions of as many genes as possible of a given organism. It combines different -omics techniques such as transcriptomics and proteomics with saturated mutant collections.[292][293]
Many omes beyond the original genome have become useful and have been widely adopted in recent years by research scientists. The suffix -omics can provide an easy shorthand to encapsulate a field; for example, an interactomics study is reasonably recognisable as relating to large-scale analyses of gene-gene, protein-protein, or protein-ligand interactions, while proteomics has become established as a term for studying proteins on a large scale.
Any given omics technique, used just by itself, cannot adequately disentangle the intricacies of a host microbiome. Multi-omics approaches are needed to satisfactorily unravel the complexities of the host-microbiome interactions.[294] For instance, metagenomics, metatranscriptomics, metaproteomics and metabolomics methods are all used to provide information on the metagenome.[295]


See...
- Brüwer, J.D. and Buck-Wiese, H. (2018) "Reading the Book of Life–Omics as a Universal Tool Across Disciplines". In: YOUMARES 8 – Oceans Across Boundaries: Learning from each other, pages 73–82. Springer. ISBN:9783319932842.
Anthropogenic impacts

In marine environments, microbial primary production contributes substantially to CO2 sequestration. Marine microorganisms also recycle nutrients for use in the marine food web and in the process release CO2 to the atmosphere. Microbial biomass and other organic matter (remnants of plants and animals) are converted to fossil fuels over millions of years. By contrast, burning of fossil fuels liberates greenhouse gases in a small fraction of that time. As a result, the carbon cycle is out of balance, and atmospheric CO2 levels will continue to rise as long as fossil fuels continue to be burnt.[6]
History
Marine microorganisms known as cyanobacteria first emerged in the oceans during the Precambrian era roughly 2 billion years ago. Over eons, the photosynthesis of marine microorganisms generated by oxygen has helped shape the chemical environment in the evolution of plants, animals and many other life forms. Marine microorganisms were first observed in 1675 by Dutch lensmaker Antonie van Leeuwenhoek.
See also
- Marine life
- Deep chlorophyll maximum
- Hawaii Ocean Time-series
- International Census of Marine Microbes
- Marine microbial symbiosis
- Microbial biogeography
- Microbial communities
- Microbial ecology
- Microbial food web
- Microbial loop
- Microbial oxidation of sulfur
- Microbially induced sedimentary structure
- Microbiology of oxygen minimum zones
- Oceanic carbon cycle
- Ooid
- Picoeukaryote
- Roseobacter
References
- ↑ 1.0 1.1 Brock Biology of Microorganisms (13th ed.). Pearson Education. 2006. p. 1096. ISBN 978-0-321-73551-5.
- ↑ 2.0 2.1 Rybicki EP (1990). "The classification of organisms at the edge of life, or problems with virus systematics". South African Journal of Science 86: 182–6. ISSN 0038-2353.
- ↑ 3.0 3.1 Lwoff A (1956). "The concept of virus". Journal of General Microbiology 17 (2): 239–53. doi:10.1099/00221287-17-2-239. PMID 13481308.
- ↑ 4.0 4.1 4.2 Bar-On, YM; Phillips, R; Milo, R (2018). "The biomass distribution on Earth". PNAS 115 (25): 6506–6511. doi:10.1073/pnas.1711842115. PMID 29784790. Bibcode: 2018PNAS..115.6506B.
- ↑ Census Of Marine Life Accessed 29 October 2020.
- ↑ 6.0 6.1 6.2 6.3 6.4 Cavicchioli, Ricardo; Ripple, William J.; Timmis, Kenneth N.; Azam, Farooq; Bakken, Lars R.; Baylis, Matthew; Behrenfeld, Michael J.; Boetius, Antje et al. (2019). "Scientists' warning to humanity: Microorganisms and climate change". Nature Reviews Microbiology 17 (9): 569–586. doi:10.1038/s41579-019-0222-5. PMID 31213707.
 Modified text was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
Modified text was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
- ↑ Bolhuis, H. and Cretoiu, M.S. (2016) "What is so special about marine microorganisms?". In: L. J. Stal and M. S. Cretoiu (Eds.) The Marine Microbiome, pages 3–20, Springer. ISBN:9783319330006
- ↑ 8.0 8.1 "Functions of global ocean microbiome key to understanding environmental changes". University of Georgia. 10 December 2015. https://www.sciencedaily.com/releases/2015/12/151210181647.htm.
- ↑ 9.0 9.1 2002 WHO mortality data Accessed 20 January 2007
- ↑ Wade, Nicholas (25 July 2016). "Meet Luca, the Ancestor of All Living Things". New York Times. https://www.nytimes.com/2016/07/26/science/last-universal-ancestor.html.
- ↑ Suttle, C.A. (2005). "Viruses in the Sea". Nature 437 (9): 356–361. doi:10.1038/nature04160. PMID 16163346. Bibcode: 2005Natur.437..356S.
- ↑ 12.0 12.1 Glöckner, F.O., Gasol, J.M., McDonough, N., Calewaert, J.B. et al. (2012) Marine microbial diversity and its role in ecosystem functioning and environmental change. European Science Foundation, Position Paper 17. ISBN 978-2-918428-71-8
- ↑ Suttle, C.A. (2005). "Viruses in the Sea". Nature 437 (9): 356–361. doi:10.1038/nature04160. PMID 16163346. Bibcode: 2005Natur.437..356S.
- ↑ Shors p. 5
- ↑ Shors p. 593
- ↑ 16.0 16.1 16.2 Suttle CA (2007). "Marine viruses—major players in the global ecosystem". Nature Reviews Microbiology 5 (10): 801–12. doi:10.1038/nrmicro1750. PMID 17853907.
- ↑ Living Bacteria Are Riding Earth's Air Currents Smithsonian Magazine, 11 January 2016.
- ↑ Robbins, Jim (13 April 2018). "Trillions Upon Trillions of Viruses Fall From the Sky Each Day". The New York Times. https://www.nytimes.com/2018/04/13/science/virosphere-evolution.html.
- ↑ Reche, Isabel; D'Orta, Gaetano; Mladenov, Natalie; Winget, Danielle M; Suttle, Curtis A (29 January 2018). "Deposition rates of viruses and bacteria above the atmospheric boundary layer". ISME Journal 12 (4): 1154–1162. doi:10.1038/s41396-017-0042-4. PMID 29379178. Bibcode: 2018ISMEJ..12.1154R.
- ↑ Staff (2014). "The Biosphere". Aspen Global Change Institute. http://www.agci.org/classroom/biosphere/index.php.
- ↑ 21.0 21.1 21.2 Choi, Charles Q. (17 March 2013). "Microbes Thrive in Deepest Spot on Earth". LiveScience. http://www.livescience.com/27954-microbes-mariana-trench.html.
- ↑ Glud, Ronnie; Wenzhöfer, Frank; Middelboe, Mathias; Oguri, Kazumasa; Turnewitsch, Robert; Canfield, Donald E.; Kitazato, Hiroshi (17 March 2013). "High rates of microbial carbon turnover in sediments in the deepest oceanic trench on Earth". Nature Geoscience 6 (4): 284–288. doi:10.1038/ngeo1773. Bibcode: 2013NatGe...6..284G.
- ↑ Oskin, Becky (14 March 2013). "Intraterrestrials: Life Thrives in Ocean Floor". LiveScience. http://www.livescience.com/27899-ocean-subsurface-ecosystem-found.html.
- ↑ Morelle, Rebecca (15 December 2014). "Microbes discovered by deepest marine drill analysed". BBC News. https://www.bbc.com/news/science-environment-30489814.
- ↑ Takai K; Nakamura K; Toki T; Tsunogai U et al. (2008). "Cell proliferation at 122°C and isotopically heavy CH4 production by a hyperthermophilic methanogen under high-pressure cultivation". Proceedings of the National Academy of Sciences of the United States of America 105 (31): 10949–54. doi:10.1073/pnas.0712334105. PMID 18664583. Bibcode: 2008PNAS..10510949T.
- ↑ Fox, Douglas (20 August 2014). "Lakes under the ice: Antarctica's secret garden". Nature 512 (7514): 244–246. doi:10.1038/512244a. PMID 25143097. Bibcode: 2014Natur.512..244F.
- ↑ Mack, Eric (20 August 2014). "Life Confirmed Under Antarctic Ice; Is Space Next?". Forbes. https://www.forbes.com/sites/ericmack/2014/08/20/life-confirmed-under-antarctic-ice-is-space-next/.
- ↑ Armbrust, E.V.; Palumbi, S.R. (2015). "Uncovering hidden worlds of ocean biodiversity". Science 348 (6237): 865–867. doi:10.1126/science.aaa7378. PMID 25999494. Bibcode: 2015Sci...348..865A.
- ↑ Azam, F.; Malfatti, F. (2007). "Microbial structuring of marine ecosystems". Nature Reviews Microbiology 5 (10): 782–791. doi:10.1038/nrmicro1747. PMID 17853906.
- ↑ Middelboe, M.; Brussaard, C. (2017). "Marine viruses: key players in marine ecosystems". Viruses 9 (10): 302. doi:10.3390/v9100302. PMID 29057790.
- ↑ Koonin EV, Senkevich TG, Dolja VV (2006). "The ancient Virus World and evolution of cells". Biology Direct 1: 29. doi:10.1186/1745-6150-1-29. PMID 16984643.
- ↑ 32.0 32.1 Canchaya C, Fournous G, Chibani-Chennoufi S, Dillmann ML, Brüssow H (2003). "Phage as agents of lateral gene transfer". Current Opinion in Microbiology 6 (4): 417–24. doi:10.1016/S1369-5274(03)00086-9. PMID 12941415.
- ↑ 33.0 33.1 Rybicki, EP (1990). "The classification of organisms at the edge of life, or problems with virus systematics". South African Journal of Science 86: 182–186.
- ↑ Koonin, E. V.; Starokadomskyy, P. (October 2016). "Are viruses alive? The replicator paradigm sheds decisive light on an old but misguided question". Studies in History and Philosophy of Science Part C: Studies in History and Philosophy of Biological and Biomedical Sciences 59: 125–134. doi:10.1016/j.shpsc.2016.02.016. PMID 26965225.
- ↑ Iyer LM, Balaji S, Koonin EV, Aravind L (2006). "Evolutionary genomics of nucleo-cytoplasmic large DNA viruses". Virus Research 117 (1): 156–84. doi:10.1016/j.virusres.2006.01.009. PMID 16494962. https://zenodo.org/record/1259447.
- ↑ Sanjuán R, Nebot MR, Chirico N, Mansky LM, Belshaw R (October 2010). "Viral mutation rates". Journal of Virology 84 (19): 9733–48. doi:10.1128/JVI.00694-10. PMID 20660197.
- ↑ Mahy WJ & Van Regenmortel MHV (eds) (2009). Desk Encyclopedia of General Virology. Oxford: Academic Press. pp. 28. ISBN 978-0-12-375146-1.
- ↑ "Are viruses alive? The replicator paradigm sheds decisive light on an old but misguided question". Studies in History and Philosophy of Biological and Biomedical Sciences 59: 125–134. 2016. doi:10.1016/j.shpsc.2016.02.016. PMID 26965225.
- ↑ Koonin, E. V.; Starokadomskyy, P. (7 March 2016). "Are viruses alive? The replicator paradigm sheds decisive light on an old but misguided question.". Studies in History and Philosophy of Biological and Biomedical Sciences 59: 125–34. doi:10.1016/j.shpsc.2016.02.016. PMID 26965225.
- ↑ 40.0 40.1 Mann, NH (2005-05-17). "The Third Age of Phage". PLOS Biology 3 (5): 753–755. doi:10.1371/journal.pbio.0030182. PMID 15884981.
- ↑ Wommack KE, Colwell RR (2000). "Virioplankton: viruses in aquatic ecosystems". Microbiology and Molecular Biology Reviews 64 (1): 69–114. doi:10.1128/MMBR.64.1.69-114.2000. PMID 10704475.
- ↑ Suttle CA (2005). "Viruses in the sea". Nature 437 (7057): 356–361. doi:10.1038/nature04160. PMID 16163346. Bibcode: 2005Natur.437..356S.
- ↑ Bergh O, Børsheim KY, Bratbak G, Heldal M (1989). "High abundance of viruses found in aquatic environments". Nature 340 (6233): 467–8. doi:10.1038/340467a0. PMID 2755508. Bibcode: 1989Natur.340..467B.
- ↑ Wigington CH, Sonderegger D, Brussaard CPD, Buchan A, Finke JF, Fuhrman JA, Lennon JT, Middelboe M, Suttle CA, Stock C, Wilson WH, Wommack KE, Wilhelm SW, Weitz JS (2016). "Re-examination of the relationship between marine virus and microbial cell abundances". Nature Microbiology 1 (3): 15024. doi:10.1038/nmicrobiol.2015.24. PMID 27572161. http://www.vliz.be/imisdocs/publications/23/301523.pdf.
- ↑ "Global morphological analysis of marine viruses shows minimal regional variation and dominance of non-tailed viruses". The ISME Journal 7 (9): 1738–51. September 2013. doi:10.1038/ismej.2013.67. PMID 23635867. Bibcode: 2013ISMEJ...7.1738B.
- ↑ "Putative prophages related to lytic tailless marine dsDNA phage PM2 are widespread in the genomes of aquatic bacteria". BMC Genomics 8: 236. 2007. doi:10.1186/1471-2164-8-236. PMID 17634101.
- ↑ "High frequency of a novel filamentous phage, VCY φ, within an environmental Vibrio cholerae population". Applied and Environmental Microbiology 78 (1): 28–33. 2012. doi:10.1128/AEM.06297-11. PMID 22020507. Bibcode: 2012ApEnM..78...28X.
- ↑ "Evolution and diversity of the Microviridae viral family through a collection of 81 new complete genomes assembled from virome reads". PLOS ONE 7 (7): e40418. 2012. doi:10.1371/journal.pone.0040418. PMID 22808158. Bibcode: 2012PLoSO...740418R.
- ↑ Kauffman, Kathryn M.; Hussain, Fatima A.; Yang, Joy; Arevalo, Philip; Brown, Julia M.; Chang, William K.; VanInsberghe, David; Elsherbini, Joseph et al. (2018). "A major lineage of non-tailed dsDNA viruses as unrecognized killers of marine bacteria". Nature 554 (7690): 118–122. doi:10.1038/nature25474. PMID 29364876. Bibcode: 2018Natur.554..118K. https://www.nature.com/articles/nature25474.
- ↑ Scientists Find New Type of Virus in World's Oceans: Autolykiviridae, on: sci-news, 25 January 2018
- ↑ Never-Before-Seen Viruses With Weird DNA Were Just Discovered in The Ocean, on: sciencealert, 25 January 2018
- ↑ NCBI: Autolykiviridae (family) – unclassified dsDNA viruses
- ↑ Lawrence CM, Menon S, Eilers BJ (2009). "Structural and functional studies of archaeal viruses". Journal of Biological Chemistry 284 (19): 12599–603. doi:10.1074/jbc.R800078200. PMID 19158076.
- ↑ Prangishvili D, Forterre P, Garrett RA (2006). "Viruses of the Archaea: a unifying view". Nature Reviews Microbiology 4 (11): 837–48. doi:10.1038/nrmicro1527. PMID 17041631.
- ↑ Prangishvili D, Garrett RA (2004). "Exceptionally diverse morphotypes and genomes of crenarchaeal hyperthermophilic viruses". Biochemical Society Transactions 32 (Pt 2): 204–8. doi:10.1042/BST0320204. PMID 15046572. https://curis.ku.dk/ws/files/51497971/0320204.pdf.
- ↑ Suttle CA (2005). "Viruses in the sea". Nature 437 (7057): 356–61. doi:10.1038/nature04160. PMID 16163346. Bibcode: 2005Natur.437..356S.
- ↑ "Harmful Algal Blooms: Red Tide: Home {{{!}}} CDC HSB". www.cdc.gov. https://www.cdc.gov/hab/redtide/.
- ↑ Forterre P, Philippe H (1999). "The last universal common ancestor (LUCA), simple or complex?". The Biological Bulletin 196 (3): 373–5; discussion 375–7. doi:10.2307/1542973. PMID 11536914.
- ↑ Abrahão, Jônatas; Silva, Lorena; Silva, Ludmila Santos; Khalil, Jacques Yaacoub Bou; Rodrigues, Rodrigo; Arantes, Thalita; Assis, Felipe; Boratto, Paulo et al. (27 February 2018). "Tailed giant Tupanvirus possesses the most complete translational apparatus of the known virosphere". Nature Communications 9 (1): 749. doi:10.1038/s41467-018-03168-1. PMID 29487281. Bibcode: 2018NatCo...9..749A.
- ↑ Reardon, Sara (2017-04-06). "Patchwork giant viruses reignite debate over origin of viruses Patchwork giant viruses reignite debate over domains of life Patchwork giant viruses spark debate over the tree of life" (in en). Nature. doi:10.1038/nature.2017.21798. ISSN 1476-4687. https://www.nature.com/articles/nature.2017.21798.
- ↑ Bichell, Rae Ellen. "In Giant Virus Genes, Hints About Their Mysterious Origin". All Things Considered. https://www.npr.org/sections/health-shots/2017/04/06/522478901/in-giant-virus-genes-hints-about-their-mysterious-origin.
- ↑ Van Etten, James L. (July–August 2011). "Giant Viruses". American Scientist 99 (4): 304–311. doi:10.1511/2011.91.304. http://www.americanscientist.org/issues/feature/2011/4/giant-viruses.
- ↑ "Genomics of Megavirus and the elusive fourth domain of Life". Communicative & Integrative Biology 5 (1): 102–6. January 2012. doi:10.4161/cib.18624. PMID 22482024.
- ↑ "Giant viruses with an expanded complement of translation system components". Science 356 (6333): 82–85. April 2017. doi:10.1126/science.aal4657. PMID 28386012. Bibcode: 2017Sci...356...82S. https://escholarship.org/content/qt0kf9t6gn/qt0kf9t6gn.pdf?t=oruwia.
- ↑ "Virus Genomes from Deep Sea Sediments Expand the Ocean Megavirome and Support Independent Origins of Viral Gigantism". mBio 10 (2): e02497-02418. March 2019. doi:10.1128/mBio.02497-18. PMID 30837339.
- ↑ "Geomicrobiology of high-level nuclear waste-contaminated vadose sediments at the Hanford site, Washington state". Applied and Environmental Microbiology 70 (7): 4230–41. 2004. doi:10.1128/AEM.70.7.4230-4241.2004. PMID 15240306. Bibcode: 2004ApEnM..70.4230F.
- ↑ "Towards a natural system of organisms: proposal for the domains Archaea, Bacteria, and Eucarya". Proceedings of the National Academy of Sciences of the United States of America 87 (12): 4576–9. 1990. doi:10.1073/pnas.87.12.4576. PMID 2112744. Bibcode: 1990PNAS...87.4576W.
- ↑ "Disparate rates, differing fates: tempo and mode of evolution changed from the Precambrian to the Phanerozoic". Proceedings of the National Academy of Sciences of the United States of America 91 (15): 6735–42. 1994. doi:10.1073/pnas.91.15.6735. PMID 8041691. Bibcode: 1994PNAS...91.6735S.
- ↑ "Environmental diversity of bacteria and archaea". Systematic Biology 50 (4): 470–8. 2001. doi:10.1080/106351501750435040. PMID 12116647.
- ↑ "Archaea and the prokaryote-to-eukaryote transition". Microbiology and Molecular Biology Reviews 61 (4): 456–502. 1997. doi:10.1128/mmbr.61.4.456-502.1997. PMID 9409149.
- ↑ Dyall, Sabrina D.; Brown, Mark T.; Johnson, Patricia J. (9 April 2004). "Ancient Invasions: From Endosymbionts to Organelles". Science 304 (5668): 253–257. doi:10.1126/science.1094884. ISSN 0036-8075. PMID 15073369. Bibcode: 2004Sci...304..253D.
- ↑ "Evaluating hypotheses for the origin of eukaryotes". BioEssays 29 (1): 74–84. 2007. doi:10.1002/bies.20516. PMID 17187354.
- ↑ "Mitochondrial genome evolution and the origin of eukaryotes". Annual Review of Genetics 33: 351–97. 1999. doi:10.1146/annurev.genet.33.1.351. PMID 10690412.
- ↑ "Endosymbiosis and evolution of the plant cell". Current Opinion in Plant Biology 2 (6): 513–9. 1999. doi:10.1016/S1369-5266(99)00025-4. PMID 10607659.
- ↑ Patrick J. Keeling (2004). "Diversity and evolutionary history of plastids and their hosts". American Journal of Botany 91 (10): 1481–1493. doi:10.3732/ajb.91.10.1481. PMID 21652304.
- ↑ "Candidatus Pelagibacter Ubique." European Bioinformatics Institute. European Bioinformatics Institute, 2011. Web. 8 Jan. 2012. http://www.ebi.ac.uk/2can/genomes/bacteria/Candidatus_Pelagibacter_ubique.html
- ↑ "Flea market: A newly discovered virus may be the most abundant organism on the planet". The Economist. 16 February 2013. https://www.economist.com/news/science-and-technology/21571843-newly-discovered-virus-may-be-most-abundant-organism-planet-flea.
- ↑ Zhao, Y.; Temperton, B.; Thrash, J. C.; Schwalbach, M. S.; Vergin, K. L.; Landry, Z. C.; Ellisman, M.; Deerinck, T. et al. (2013). "Abundant SAR11 viruses in the ocean". Nature 494 (7437): 357–360. doi:10.1038/nature11921. PMID 23407494. Bibcode: 2013Natur.494..357Z.
- ↑ "The largest Bacterium: Scientist discovers new bacterial life form off the African coast", Max Planck Institute for Marine Microbiology, 8 April 1999, http://www.mpg.de/english/illustrationsDocumentation/documentation/pressReleases/1999/news17_99.htm
- ↑ List of Prokaryotic names with Standing in Nomenclature – Genus Thiomargarita, https://lpsn.dsmz.de/genus/thiomargarita
- ↑ "Archaea associated with human surfaces: not to be underestimated". FEMS Microbiology Reviews 39 (5): 631–48. 2015. doi:10.1093/femsre/fuv010. PMID 25907112.
- ↑ Archaea Online Etymology Dictionary. Retrieved 17 August 2016.
- ↑ Pace NR (May 2006). "Time for a change". Nature 441 (7091): 289. doi:10.1038/441289a. PMID 16710401. Bibcode: 2006Natur.441..289P.
- ↑ Stoeckenius W (1 October 1981). "Walsby's square bacterium: fine structure of an orthogonal procaryote". Journal of Bacteriology 148 (1): 352–60. doi:10.1128/JB.148.1.352-360.1981. PMID 7287626.
- ↑ Madigan M and Martinko J (Eds.) (2005). Brock Biology of Microorganisms (11th ed.). Prentice Hall. ISBN 978-0-13-144329-7.
- ↑ "Deep sea microorganisms and the origin of the eukaryotic cell". http://protistology.jp/journal/jjp47/JJP47YAMAGUCHI.pdf.
- ↑ Yamaguchi, Masashi (1 December 2012). "Prokaryote or eukaryote? A unique microorganism from the deep sea". Journal of Electron Microscopy 61 (6): 423–431. doi:10.1093/jmicro/dfs062. PMID 23024290.
- ↑ Whittaker, R.H.; Margulis, L. (1978). "Protist classification and the kingdoms of organisms". Biosystems 10 (1–2): 3–18. doi:10.1016/0303-2647(78)90023-0. PMID 418827. Bibcode: 1978BiSys..10....3W.
- ↑ Faure, E; Not, F; Benoiston, AS; Labadie, K; Bittner, L; Ayata, SD (2019). "Mixotrophic protists display contrasted biogeographies in the global ocean". ISME Journal 13 (4): 1072–1083. doi:10.1038/s41396-018-0340-5. PMID 30643201. Bibcode: 2019ISMEJ..13.1072F.
- ↑ 90.0 90.1 90.2 Leles, S.G.; Mitra, A.; Flynn, K.J.; Stoecker, D.K.; Hansen, P.J.; Calbet, A.; McManus, G.B.; Sanders, R.W. et al. (2017). "Oceanic protists with different forms of acquired phototrophy display contrasting biogeographies and abundance". Proceedings of the Royal Society B: Biological Sciences 284 (1860): 20170664. doi:10.1098/rspb.2017.0664. PMID 28768886.
- ↑ Budd, Graham E; Jensen, Sören (2017). "The origin of the animals and a 'Savannah' hypothesis for early bilaterian evolution". Biological Reviews 92 (1): 446–473. doi:10.1111/brv.12239. PMID 26588818.
- ↑ Cavalier-Smith T (December 1993). "Kingdom protozoa and its 18 phyla". Microbiological Reviews 57 (4): 953–94. doi:10.1128/mmbr.57.4.953-994.1993. PMID 8302218.
- ↑ Corliss JO (1992). "Should there be a separate code of nomenclature for the protists?". BioSystems 28 (1–3): 1–14. doi:10.1016/0303-2647(92)90003-H. PMID 1292654. Bibcode: 1992BiSys..28....1C.
- ↑ "The extent of protist diversity: insights from molecular ecology of freshwater eukaryotes". Proceedings of the Royal Society B: Biological Sciences 272 (1576): 2073–81. 2005. doi:10.1098/rspb.2005.3195. PMID 16191619.
- ↑ "The molecular ecology of microbial eukaryotes unveils a hidden world". Trends in Microbiology 10 (1): 31–8. 2002. doi:10.1016/S0966-842X(01)02257-0. PMID 11755083. http://download.bioon.com.cn/view/upload/month_0803/20080326_daa08a6fdb5d38e3a0d8VBrocN3WtOdR.attach.pdf.
- ↑ 96.0 96.1 The Air You're Breathing? A Diatom Made That
- ↑ "More on Diatoms". http://www.ucmp.berkeley.edu/chromista/diatoms/diatommm.html.
- ↑ Devreotes P (1989). "Dictyostelium discoideum: a model system for cell-cell interactions in development". Science 245 (4922): 1054–8. doi:10.1126/science.2672337. PMID 2672337. Bibcode: 1989Sci...245.1054D.
- ↑ Matz, Mikhail V.; Tamara M. Frank; N. Justin Marshall; Edith A. Widder; Sonke Johnsen (2008-12-09). "Giant Deep-Sea Protist Produces Bilaterian-like Traces". Current Biology (Elsevier Ltd) 18 (23): 1849–1854. doi:10.1016/j.cub.2008.10.028. PMID 19026540. http://www.biology.duke.edu/johnsenlab/pdfs/pubs/sea%20grapes%202008.pdf.
- ↑ Gooday, A. J.; Aranda da Silva, A.; Pawlowski, J. (2011-12-01). "Xenophyophores (Rhizaria, Foraminifera) from the Nazaré Canyon (Portuguese margin, NE Atlantic)". Deep-Sea Research Part II: Topical Studies in Oceanography. The Geology, Geochemistry, and Biology of Submarine Canyons West of Portugal 58 (23–24): 2401–2419. doi:10.1016/j.dsr2.2011.04.005. Bibcode: 2011DSRII..58.2401G.
- ↑ Neil A C, Reece J B, Simon E J (2004) Essential biology with physiology Pearson/Benjamin Cummings, Page 291. ISBN:9780805375039
- ↑ "The other eukaryotes in light of evolutionary protistology". Biology & Philosophy 28 (2): 299–330. 2012. doi:10.1007/s10539-012-9354-y.
- ↑ "The new higher level classification of eukaryotes with emphasis on the taxonomy of protists". The Journal of Eukaryotic Microbiology 52 (5): 399–451. 2005. doi:10.1111/j.1550-7408.2005.00053.x. PMID 16248873. http://doc.rero.ch/record/14409/files/PAL_E1847.pdf.
- ↑ Margulis, Lynn; Chapman, Michael J. (2009-03-19). Kingdoms and Domains: An Illustrated Guide to the Phyla of Life on Earth. Academic Press. ISBN 9780080920146. https://books.google.com/books?id=9IWaqAOGyt4C.
- ↑ 105.0 105.1 Dawson, Scott C; Paredez, Alexander R (2013). "Alternative cytoskeletal landscapes: cytoskeletal novelty and evolution in basal excavate protists". Current Opinion in Cell Biology 25 (1): 134–141. doi:10.1016/j.ceb.2012.11.005. PMID 23312067.
- ↑ 106.0 106.1 Atkinson, A.; Polimene, L.; Fileman, E.S.; Widdicombe, C.E.; McEvoy, A.J.; Smyth, T.J.; Djeghri, N.; Sailley, S.F. et al. (2018). ""Comment. What drives plankton seasonality in a stratifying shelf sea? Some competing and complementary theories"". Limnology and Oceanography 63 (6): 2877–2884. doi:10.1002/lno.11036. Bibcode: 2018LimOc..63.2877A. http://plymsea.ac.uk/id/eprint/8073/1/Kenitz%20comment_revised%20ACCEPTED.pdf.
- ↑ Singleton, Paul (2006). Dictionary of Microbiology and Molecular Biology, 3rd Edition, revised. Chichester, UK: John Wiley & Sons. pp. 32. ISBN 978-0-470-03545-0.
- ↑ David J. Patterson. "Amoebae: Protists Which Move and Feed Using Pseudopodia". Tree of Life web project. http://tolweb.org/notes/?note_id=51.
- ↑ "The Amoebae". The University of Edinburgh. http://www.bms.ed.ac.uk/research/others/smaciver/amoebae.htm.
- ↑ Brady, Richard M. (1993). "Torque and switching in the bacterial flagellar motor. An electrostatic model". Biophysical Journal 64 (4): 961–973. doi:10.1016/S0006-3495(93)81462-0. PMID 7684268. Bibcode: 1993BpJ....64..961B.
- ↑ Lauga, Eric; Thomas R Powers (25 August 2009). "The hydrodynamics of swimming microorganisms". Reports on Progress in Physics 72 (9): 096601. doi:10.1088/0034-4885/72/9/096601. Bibcode: 2009RPPh...72i6601L.
- ↑ Hyde, K.D.; E.B.J. Jones; E. Leaño; S.B. Pointing; A.D. Poonyth; L.L.P. Vrijmoed (1998). "Role of fungi in marine ecosystems". Biodiversity and Conservation 7 (9): 1147–1161. doi:10.1023/A:1008823515157. Bibcode: 1998BiCon...7.1147H.
- ↑ Kirk, P.M., Cannon, P.F., Minter, D.W. and Stalpers, J. "Dictionary of the Fungi". Edn 10. CABI, 2008
- ↑ Hyde, K.D.; E.B.J. Jones (1989). "Spore attachment in marine fungi". Botanica Marina 32 (3): 205–218. doi:10.1515/botm.1989.32.3.205.
- ↑ "Fungal diversity in deep-sea hydrothermal ecosystems". Applied and Environmental Microbiology 75 (20): 6415–21. October 2009. doi:10.1128/AEM.00653-09. PMID 19633124. Bibcode: 2009ApEnM..75.6415L.
- ↑ San-Martín, A.; S. Orejanera; C. Gallardo; M. Silva; J. Becerra; R. Reinoso; M.C. Chamy; K. Vergara et al. (2008). "Steroids from the marine fungus Geotrichum sp". Journal of the Chilean Chemical Society 53 (1): 1377–1378. doi:10.4067/S0717-97072008000100011.
- ↑ Jones, E.B.G., Hyde, K.D., & Pang, K.-L., eds. (2014). Freshwater fungi: and fungal-like organisms. Berlin/Boston: De Gruyter.
- ↑ Jones, E.B.G.; Pang, K.-L., eds (2012). Marine Fungi, and Fungal-like Organisms. Marine and Freshwater Botany. Berlin, Boston: De Gruyter (published August 2012). doi:10.1515/9783110264067. ISBN 978-3-11-026406-7. http://www.degruyter.com/view/product/177990. Retrieved 3 September 2015.
- ↑ Wang, Xin; Singh, Purnima; Gao, Zheng; Zhang, Xiaobo; Johnson, Zackary I.; Wang, Guangyi (2014). "Distribution and Diversity of Planktonic Fungi in the West Pacific Warm Pool". PLOS ONE 9 (7): e101523. doi:10.1371/journal.pone.0101523.s001. PMID 24992154. Bibcode: 2014PLoSO...9j1523W.
- ↑ Wang, G.; Wang, X.; Liu, X.; Li, Q. (2012). "Diversity and biogeochemical function of planktonic fungi in the ocean". in Raghukumar, C.. Biology of marine fungi. Berlin, Heidelberg: Springer-Verlag. pp. 71–88. doi:10.1007/978-3-642-23342-5. ISBN 978-3-642-23341-8. https://www.springer.com/us/book/9783642233418. Retrieved 3 September 2015.
- ↑ Damare, Samir; Raghukumar, Chandralata (2007-11-11). "Fungi and Macroaggregation in Deep-Sea Sediments". Microbial Ecology 56 (1): 168–177. doi:10.1007/s00248-007-9334-y. ISSN 0095-3628. PMID 17994287.
- ↑ Kubanek, Julia; Jensen, Paul R.; Keifer, Paul A.; Sullards, M. Cameron; Collins, Dwight O.; Fenical, William (2003-06-10). "Seaweed resistance to microbial attack: A targeted chemical defense against marine fungi". Proceedings of the National Academy of Sciences 100 (12): 6916–6921. doi:10.1073/pnas.1131855100. ISSN 0027-8424. PMID 12756301. Bibcode: 2003PNAS..100.6916K.
- ↑ 123.0 123.1 Gao, Zheng; Johnson, Zackary I.; Wang, Guangyi (2009-07-30). "Molecular characterization of the spatial diversity and novel lineages of mycoplankton in Hawaiian coastal waters". The ISME Journal 4 (1): 111–120. doi:10.1038/ismej.2009.87. ISSN 1751-7362. PMID 19641535.
- ↑ Panzer, Katrin; Yilmaz, Pelin; Weiß, Michael; Reich, Lothar; Richter, Michael; Wiese, Jutta; Schmaljohann, Rolf; Labes, Antje et al. (2015-07-30). "Identification of Habitat-Specific Biomes of Aquatic Fungal Communities Using a Comprehensive Nearly Full-Length 18S rRNA Dataset Enriched with Contextual Data". PLOS ONE 10 (7): e0134377. doi:10.1371/journal.pone.0134377. PMID 26226014. Bibcode: 2015PLoSO..1034377P.
- ↑ Gutierrez, Marcelo H; Pantoja, Silvio; Quinones, Renato a and Gonzalez, Rodrigo R. First record of flamentous fungi in the coastal upwelling ecosystem off central Chile. Gayana (Concepc.) [online]. 2010, vol.74, n.1, pp. 66-73. ISSN 0717-6538.
- ↑ 126.0 126.1 Sridhar, K.R. (2009). "10. Aquatic fungi – Are they planktonic?". Plankton Dynamics of Indian Waters. Jaipur, India: Pratiksha Publications. pp. 133–148.
- ↑ Species of Higher Marine Fungi University of Mississippi. Retrieved 2012-02-05.
- ↑ Freshwater and marine lichen-forming fungi Retrieved 2012-02-06.
- ↑ "Lichens". National Park Service, US Department of the Interior, Government of the United States. 22 May 2016. https://www.nps.gov/glac/learn/nature/lichens.htm.
- ↑ 130.0 130.1 "The Earth Life Web, Growth and Development in Lichens". earthlife.net. 14 February 2020. http://www.earthlife.net/lichens/growth.html.
- ↑ Silliman B. R.; S. Y. Newell (2003). "Fungal farming in a snail". PNAS 100 (26): 15643–15648. doi:10.1073/pnas.2535227100. PMID 14657360. Bibcode: 2003PNAS..10015643S.
- ↑ "Lichen-Like Symbiosis 600 Million Years Ago". Science 308 (5724): 1017–1020. 2005. doi:10.1126/science.1111347. PMID 15890881. Bibcode: 2005Sci...308.1017Y.
- ↑ Jones, E. B. Gareth; Pang, Ka-Lai (2012-08-31). Marine Fungi: and Fungal-like Organisms. Walter de Gruyter. ISBN 9783110264067. https://books.google.com/books?id=RcF97cHppPsC.
- ↑ Janet Fang (6 April 2010). "Animals thrive without oxygen at sea bottom". Nature 464 (7290): 825. doi:10.1038/464825b. PMID 20376121. Bibcode: 2010Natur.464..825F.
- ↑ "Briny deep basin may be home to animals thriving without oxygen". Science News. 2013-09-23. http://www.sciencenews.org/view/generic/id/58154/description/Multicelled_animals_may_live_oxygen-free.
- ↑ 136.0 136.1 Campbell, Neil A.; Reece, Jane B.; Urry, Lisa Andrea; Cain, Michael L.; Wasserman, Steven Alexander; Minorsky, Peter V.; Jackson, Robert Bradley (2008). Biology (8 ed.). San Francisco: Pearson – Benjamin Cummings. ISBN 978-0-321-54325-7.
- ↑ McNeill, J., ed (2012). International Code of Nomenclature for algae, fungi, and plants (Melbourne Code), Adopted by the Eighteenth International Botanical Congress Melbourne, Australia, July 2011 (electronic ed.). International Association for Plant Taxonomy. http://www.iapt-taxon.org/nomen/main.php?page=art3. Retrieved 2017-05-14.
- ↑ Changes in oxygen concentrations in our ocean can disrupt fundamental biological cycles Phys.org, 25 November 2019.
- ↑ "Cyanobacteria and cyanobacterial toxins". Oceans and Human Health: Risks and Remedies from the Seas. Academic Press. 2 September 2011. pp. 271–296. ISBN 978-0-08-087782-2. https://books.google.com/books?id=LMZPqW-PmFYC&pg=PA271.
- ↑ "The Rise of Oxygen" (in en-US). 30 July 2003. http://www.astrobio.net/news-exclusive/the-rise-of-oxygen/.
- ↑ Flannery, D. T.; R.M. Walter (2012). "Archean tufted microbial mats and the Great Oxidation Event: new insights into an ancient problem". Australian Journal of Earth Sciences 59 (1): 1–11. doi:10.1080/08120099.2011.607849. Bibcode: 2012AuJES..59....1F.
- ↑ Rothschild, Lynn (September 2003). "Understand the evolutionary mechanisms and environmental limits of life". NASA. http://astrobiology.arc.nasa.gov/roadmap/g5.html.
- ↑ "The cells that rule the seas". Scientific American 289 (6): 52–3. December 2003. doi:10.1038/scientificamerican1203-52. PMID 14631732. Bibcode: 2003SciAm.289f..52N. http://guowei.ccps.tp.edu.tw/nc/UploadDocument/255_02%20The%20Cells%20that%20Rules%20the%20Sea.pdf. Retrieved 2 June 2019.
- ↑ "The Most Important Microbe You've Never Heard Of". https://www.npr.org/templates/story/story.php?storyId=91448837.
- ↑ Flombaum, P.; Gallegos, J. L.; Gordillo, R. A.; Rincon, J.; Zabala, L. L.; Jiao, N.; Karl, D. M.; Li, W. K. W. et al. (2013). "Present and future global distributions of the marine Cyanobacteria Prochlorococcus and Synechococcus". Proceedings of the National Academy of Sciences 110 (24): 9824–9829. doi:10.1073/pnas.1307701110. PMID 23703908. Bibcode: 2013PNAS..110.9824F.
- ↑ Nabors, Murray W. (2004). Introduction to Botany. San Francisco, CA: Pearson Education, Inc. ISBN 978-0-8053-4416-5.
- ↑ Allaby, M., ed (1992). "Algae". The Concise Dictionary of Botany. Oxford: Oxford University Press.
- ↑ "How many species of algae are there?". Journal of Phycology 48 (5): 1057–63. October 2012. doi:10.1111/j.1529-8817.2012.01222.x. PMID 27011267. Bibcode: 2012JPcgy..48.1057G.
- ↑ 149.0 149.1 Guiry, M.D.; Guiry, G.M. (2016). "Algaebase". http://www.algaebase.org/browse/taxonomy/?id=97240.
- ↑ D. Thomas (2002). Seaweeds. Life Series. Natural History Museum, London. ISBN 978-0-565-09175-0.
- ↑ Hoek, Christiaan; den Hoeck, Hoeck Van; Mann, David; Jahns, H.M. (1995). Algae : an introduction to phycology. Cambridge University Press. p. 166. ISBN 9780521316873. OCLC 443576944. https://books.google.com/books?id=s1P855ZWc0kC&pg=166.
- ↑ Starckx, Senne (31 October 2012) A place in the sun - Algae is the crop of the future, according to researchers in Geel Flanders Today, Retrieved 8 December 2012
- ↑ Duval, B.; Margulis, L. (1995). "The microbial community of Ophrydium versatile colonies: endosymbionts, residents, and tenants". Symbiosis 18: 181–210. PMID 11539474.
- ↑ Mandoli, DF (1998). "Elaboration of Body Plan and Phase Change during Development of Acetabularia: How Is the Complex Architecture of a Giant Unicell Built?". Annual Review of Plant Physiology and Plant Molecular Biology 49: 173–198. doi:10.1146/annurev.arplant.49.1.173. PMID 15012232.
- ↑ Pierre Madl; Maricela Yip (2004). "Literature Review of Caulerpa taxifolia". BUFUS-Info 19 (31). http://biophysics.sbg.ac.at/ct/caulerpa.htm. Retrieved 11 October 2019.
- ↑ Lalli, C.; Parsons, T. (1993). Biological Oceanography: An Introduction. Butterworth-Heinemann. ISBN 0-7506-3384-0.
- ↑ Eerie red glow traces ocean plant health NASA Science, 28 May 2009.
- ↑ Behrenfeld, M.J.; Westberry, T.K.; Boss, E.S.; O'Malley, R.T.; Siegel, D.A.; Wiggert, J.D.; Franz, B.A.; McLain, C.R. et al. (2009). "Satellite-detected fluorescence reveals global physiology of ocean phytoplankton". Biogeosciences 6 (5): 779–794. doi:10.5194/bg-6-779-2009. Bibcode: 2009BGeo....6..779B.
- ↑ 159.0 159.1 Wassilieff, Maggy (2006) "Plankton – Plant plankton", Te Ara – the Encyclopedia of New Zealand. Accessed: 2 November 2019.
- ↑ Lindsey, R., Scott, M. and Simmon, R. (2010) "What are phytoplankton". NASA Earth Observatory.
- ↑ Field, C. B.; Behrenfeld, M. J.; Randerson, J. T.; Falwoski, P. G. (1998). "Primary production of the biosphere: Integrating terrestrial and oceanic components". Science 281 (5374): 237–240. doi:10.1126/science.281.5374.237. PMID 9657713. Bibcode: 1998Sci...281..237F. https://escholarship.org/uc/item/9gm7074q.
- ↑ Treguer, P.; Nelson, D. M.; Van Bennekom, A. J.; Demaster, D. J.; Leynaert, A.; Queguiner, B. (1995). "The Silica Balance in the World Ocean: A Reestimate". Science 268 (5209): 375–9. doi:10.1126/science.268.5209.375. PMID 17746543. Bibcode: 1995Sci...268..375T.
- ↑ "King's College London – Lake Megachad" (in en-GB). https://www.kcl.ac.uk/sspp/departments/geography/people/academic/drake/Research/The-Sahara-Megalakes-Project/Lake-Megachad.aspx.
- ↑ Arsenieff, L.; Simon, N.; Rigaut-Jalabert, F.; Le Gall, F.; Chaffron, S.; Corre, E.; Com, E.; Bigeard, E. et al. (2018). "First Viruses Infecting the Marine Diatom Guinardia delicatula". Frontiers in Microbiology 9: 3235. doi:10.3389/fmicb.2018.03235. PMID 30687251.
- ↑ Zhang, D.; Wang, Y.; Cai, J.; Pan, J.; Jiang, X.; Jiang, Y. (2012). "Bio-manufacturing technology based on diatom micro- and nanostructure". Chinese Science Bulletin 57 (30): 3836–3849. doi:10.1007/s11434-012-5410-x. Bibcode: 2012ChSBu..57.3836Z.
- ↑ Wassilieff, Maggy (2006) "A coccolithophore", Te Ara – the Encyclopedia of New Zealand. Accessed: 2 November 2019.
- ↑ Rost, B. and Riebesell, U. (2004) "Coccolithophores and the biological pump: responses to environmental changes". In: Coccolithophores: From Molecular Processes to Global Impact, pages 99–125, Springer. ISBN 9783662062784.
- ↑ DeLong, E.F.; Beja, O. (2010). "The light-driven proton pump proteorhodopsin enhances bacterial survival during tough times". PLOS Biology 8 (4): e1000359. doi:10.1371/journal.pbio.1000359. e1000359. PMID 20436957.
- ↑ 169.0 169.1 Gómez-Consarnau, L.; Raven, J.A.; Levine, N.M.; Cutter, L.S.; Wang, D.; Seegers, B.; Arístegui, J.; Fuhrman, J.A. et al. (2019). "Microbial rhodopsins are major contributors to the solar energy captured in the sea". Science Advances 5 (8): eaaw8855. doi:10.1126/sciadv.aaw8855. PMID 31457093. Bibcode: 2019SciA....5.8855G.
- ↑ Oren, Aharon (2002). "Molecular ecology of extremely halophilic Archaea and Bacteria". FEMS Microbiology Ecology 39 (1): 1–7. doi:10.1111/j.1574-6941.2002.tb00900.x. ISSN 0168-6496. PMID 19709178. Bibcode: 2002FEMME..39....1O.
- ↑ Béja, O.; Aravind, L.; Koonin, E.V.; Suzuki, M.T.; Hadd, A.; Nguyen, L.P.; Jovanovich, S.B.; Gates, C.M. et al. (2000). "Bacterial rhodopsin: evidence for a new type of phototrophy in the sea". Science 289 (5486): 1902–1906. doi:10.1126/science.289.5486.1902. PMID 10988064. Bibcode: 2000Sci...289.1902B.
- ↑ "Interviews with Fellows: Ed Delong". American Academy of Microbiology. http://academy.asm.org/index.php/news-views/interviews-with-fellows/366-ed-delong.
- ↑ Bacteria with Batteries, Popular Science, January 2001, Page 55.
- ↑ Béja, O.; Aravind, L.; Koonin, E.V.; Suzuki, M.T.; Hadd, A.; Nguyen, L.P.; Jovanovich, S.B.; Gates, C.M. et al. (2000). "Bacterial rhodopsin: evidence for a new type of phototrophy in the sea". Science 289 (5486): 1902–1906. doi:10.1126/science.289.5486.1902. PMID 10988064. Bibcode: 2000Sci...289.1902B.
- ↑ Boeuf, Dominique; Audic, Stéphane; Brillet-Guéguen, Loraine; Caron, Christophe; Jeanthon, Christian (2015). "MicRhoDE: a curated database for the analysis of microbial rhodopsin diversity and evolution". Database 2015: bav080. doi:10.1093/database/bav080. ISSN 1758-0463. PMID 26286928.
- ↑ Yawo, Hiromu; Kandori, Hideki; Koizumi, Amane (5 June 2015). Optogenetics: Light-Sensing Proteins and Their Applications. Springer. pp. 3–4. ISBN 978-4-431-55516-2. https://books.google.com/books?id=5M3WCQAAQBAJ&pg=PA3. Retrieved 30 September 2015.
- ↑ A tiny marine microbe could play a big role in climate change University of Southern California, Press Room, 8 August 2019.
- ↑ DasSarma, Shiladitya; Schwieterman, Edward W. (11 October 2018). "Early evolution of purple retinal pigments on Earth and implications for exoplanet biosignatures". International Journal of Astrobiology 20 (3): 241–250. doi:10.1017/S1473550418000423. ISSN 1473-5504. Bibcode: 2018arXiv181005150D. https://www.cambridge.org/core/journals/international-journal-of-astrobiology/article/early-evolution-of-purple-retinal-pigments-on-earth-and-implications-for-exoplanet-biosignatures/63A1AD8AF544BEEF4C6D4A2D53130327.
- ↑ Sparks, William B.; DasSarma, S.; Reid, I. N. (December 2006). "Evolutionary Competition Between Primitive Photosynthetic Systems: Existence of an early purple Earth?". American Astronomical Society Meeting Abstracts 38: 901. Bibcode: 2006AAS...209.0605S.
- ↑ Redfield, Alfred C. (1934). "On the Proportions of Organic Derivatives in Sea Water and their Relation to the Composition of Plankton". in Johnstone, James; Daniel, Richard Jellicoe. James Johnstone Memorial Volume. Liverpool: University Press of Liverpool. pp. 176–92. OCLC 13993674.
- ↑ 181.0 181.1 Arrigo, Kevin R. (2005). "Marine microorganisms and global nutrient cycles". Nature 437 (7057): 349–55. doi:10.1038/nature04159. PMID 16163345. Bibcode: 2005Natur.437..349A.
- ↑ Martiny, A.C.; Pham, C.T.; Primeau, F.W.; Vrugt, J.A.; Moore, J.K.; Levin, S.A.; Lomas, M.W. (2013). "Strong latitudinal patterns in the elemental ratios of marine plankton and organic matter". Nature Geoscience 6 (4): 279–283. doi:10.1038/ngeo1757. Bibcode: 2013NatGe...6..279M. http://www.escholarship.org/uc/item/68n582hp.
- ↑ Eppley, R.W.; Peterson, B.J. (1979). "Particulate organic matter flux and planktonic new production in the deep ocean". Nature 282 (5740): 677–680. doi:10.1038/282677a0. Bibcode: 1979Natur.282..677E.
- ↑ Velicer, Gregory J.; Mendes-Soares, Helena (2007). "Bacterial predators". Cell 19 (2): R55–R56. doi:10.1016/j.cub.2008.10.043. PMID 19174136. https://www.cell.com/current-biology/pdf/S0960-9822(08)01412-7.pdf.
- ↑ Stevens, Alison N. P. (2010). "Predation, Herbivory, and Parasitism". Nature Education Knowledge 3 (10): 36. https://www.nature.com/scitable/knowledge/library/predation-herbivory-and-parasitism-13261134.
- ↑ Jurkevitch, Edouard; Davidov, Yaacov (2006). "Phylogenetic Diversity and Evolution of Predatory Prokaryotes". Predatory Prokaryotes. Springer. pp. 11–56. doi:10.1007/7171_052. ISBN 978-3-540-38577-6. https://archive.org/details/predatoryprokary00jurk.
- ↑ Hansen, Per Juel; Bjørnsen, Peter Koefoed; Hansen, Benni Winding (1997). "Zooplankton grazing and growth: Scaling within the 2-2,-μm body size range". Limnology and Oceanography 42 (4): 687–704. doi:10.4319/lo.1997.42.4.0687. Bibcode: 1997LimOc..42..687H. summarizes findings from many authors.
- ↑ 188.0 188.1 Wassilieff, Maggy (2006) "Plankton - Animal plankton", Te Ara - the Encyclopedia of New Zealand. Accessed: 2 November 2019.
- ↑ Varea, C.; Aragon, J.L.; Barrio, R.A. (1999). "Turing patterns on a sphere". Physical Review E 60 (4): 4588–92. doi:10.1103/PhysRevE.60.4588. PMID 11970318. Bibcode: 1999PhRvE..60.4588V.
- ↑ 190.0 190.1 Hemleben, C.; Anderson, O.R.; Spindler, M. (1989). Modern Planktonic Foraminifera. Springer-Verlag. ISBN 978-3-540-96815-3. https://books.google.com/books?id=NaHOmAEACAAJ.
- ↑ Foraminifera: History of Study, University College London. Retrieved: 18 November 2019.
- ↑ Advances in Microbial Ecology, Volum 11
- ↑ Bernhard, J. M.; Bowser, S.M. (1999). "Benthic Foraminifera of dysoxic sediments: chloroplast sequestration and functional morphology". Earth-Science Reviews 46 (1): 149–165. doi:10.1016/S0012-8252(99)00017-3. Bibcode: 1999ESRv...46..149B.
- ↑ Dierssen, Heidi; McManus, George B.; Chlus, Adam; Qiu, Dajun; Gao, Bo-Cai; Lin, Senjie (2015). "Space station image captures a red tide ciliate bloom at high spectral and spatial resolution". Proceedings of the National Academy of Sciences 112 (48): 14783–14787. doi:10.1073/pnas.1512538112. PMID 26627232. Bibcode: 2015PNAS..11214783D.
- ↑ Beware the mixotrophs – they can destroy entire ecosystems 'in a matter of hours'
- ↑ Microscopic body snatchers infest our oceans - Phys.org
- ↑ Eiler A (December 2006). "Evidence for the Ubiquity of Mixotrophic Bacteria in the Upper Ocean: Implications and Consequences". Appl Environ Microbiol 72 (12): 7431–7. doi:10.1128/AEM.01559-06. PMID 17028233. Bibcode: 2006ApEnM..72.7431E.
- ↑ "The mixotroph Ochromonas tuberculata may invade and suppress specialist phago- and phototroph plankton communities depending on nutrient conditions". Oecologia 148 (4): 692–701. July 2006. doi:10.1007/s00442-006-0413-4. PMID 16568278. Bibcode: 2006Oecol.148..692K.
- ↑ Schoemann, Véronique; Becquevort, Sylvie; Stefels, Jacqueline; Rousseau, Véronique; Lancelot, Christiane (2005-01-01). "Phaeocystis blooms in the global ocean and their controlling mechanisms: a review". Journal of Sea Research. Iron Resources and Oceanic Nutrients – Advancement of Global Environmental Simulations 53 (1–2): 43–66. doi:10.1016/j.seares.2004.01.008. Bibcode: 2005JSR....53...43S.
- ↑ "Welcome to the Phaeocystis antarctica genome sequencing project homepage". http://www.phaeocystis.org/.
- ↑ DiTullio, G. R.; Grebmeier, J. M.; Arrigo, K. R.; Lizotte, M. P.; Robinson, D. H.; Leventer, A.; Barry, J. P.; VanWoert, M. L. et al. (2000). "Rapid and early export of Phaeocystis antarctica blooms in the Ross Sea, Antarctica". Nature 404 (6778): 595–598. doi:10.1038/35007061. PMID 10766240. Bibcode: 2000Natur.404..595D.
- ↑ J, Stefels; L, Dijkhuizen; WWC, Gieskes (1995-07-20). "DMSP-lyase activity in a spring phytoplankton bloom off the Dutch coast, related to Phaeocystis sp. abundance". Marine Ecology Progress Series 123: 235–243. doi:10.3354/meps123235. Bibcode: 1995MEPS..123..235S. https://pure.rug.nl/ws/files/62552225/DMSP_lyase_activity_in_a_spring_phytoplankton_bloom_off_the_Dutch_coast.pdf.
- ↑ Decelle, Johan; Simó, Rafel; Galí, Martí; Vargas, Colomban de; Colin, Sébastien; Desdevises, Yves; Bittner, Lucie; Probert, Ian et al. (2012-10-30). "An original mode of symbiosis in open ocean plankton" (in en). Proceedings of the National Academy of Sciences 109 (44): 18000–18005. doi:10.1073/pnas.1212303109. ISSN 0027-8424. PMID 23071304. Bibcode: 2012PNAS..10918000D.
- ↑ Mars Brisbin, Margaret; Grossmann, Mary M.; Mesrop, Lisa Y.; Mitarai, Satoshi (2018). "Intra-host Symbiont Diversity and Extended Symbiont Maintenance in Photosymbiotic Acantharea (Clade F)" (in en). Frontiers in Microbiology 9: 1998. doi:10.3389/fmicb.2018.01998. ISSN 1664-302X. PMID 30210473.
- ↑ 205.0 205.1 Stoecker, D.K.; Hansen, P.J.; Caron, D.A.; Mitra, A. (2017). "Mixotrophy in the marine plankton". Annual Review of Marine Science 9: 311–335. doi:10.1146/annurev-marine-010816-060617. PMID 27483121. Bibcode: 2017ARMS....9..311S.
- ↑ 206.0 206.1 Mitra, AExpression error: Unrecognized word "etal". (2016). "Defining planktonic protist functional groups on mechanisms for energy and nutrient acquisition; incorporation of diverse mixotrophic strategies". Protist 167 (2): 106–20. doi:10.1016/j.protis.2016.01.003. PMID 26927496.
- ↑ Gómez F (2012). "A checklist and classification of living dinoflagellates (Dinoflagellata, Alveolata)". CICIMAR Oceánides 27 (1): 65–140. doi:10.37543/oceanides.v27i1.111.
- ↑ Stoecker, D. K. (1999). "Mixotrophy among Dinoflagellates". The Journal of Eukaryotic Microbiology 46 (4): 397–401. doi:10.1111/j.1550-7408.1999.tb04619.x.
- ↑ Suggested Explanation for Glowing Seas--Including Currently Glowing California Seas National Science Foundation, 18 October 2011.
- ↑ Boltovskoy, Demetrio; Anderson, O. Roger; Correa, Nancy M. (2017) (in en). Handbook of the Protists. Springer, Cham. pp. 731–763. doi:10.1007/978-3-319-28149-0_19. ISBN 9783319281476.
- ↑ Anderson, O. R. (1983). Radiolaria. Springer Science & Business Media.
- ↑ Gast, R. J.; Caron, D. A. (1996-11-01). "Molecular phylogeny of symbiotic dinoflagellates from planktonic foraminifera and radiolaria" (in en). Molecular Biology and Evolution 13 (9): 1192–1197. doi:10.1093/oxfordjournals.molbev.a025684. ISSN 0737-4038. PMID 8896371.
- ↑ Castro, Peter; Huber, Michael E. (2010). Marine Biology (8th ed.). McGraw Hill. pp. 95. ISBN 978-0071113021.
- ↑ "Chemistries and colors of bioluminescent reactions: a review". Gene 173 (1 Spec No): 5–11. 1996. doi:10.1016/0378-1119(95)00676-1. PMID 8707056.
- ↑ "Bioluminescence in the sea". Annual Review of Marine Science 2: 443–93. 2009. doi:10.1146/annurev-marine-120308-081028. PMID 21141672. Bibcode: 2010ARMS....2..443H.
- ↑ "Protozoa Infecting Gills and Skin". The Merck Veterinary Manual. http://www.merckvetmanual.com:80/mvm/index.jsp?cfile=htm/bc/170410.htm.
- ↑ Brand, Larry E.; Campbell, Lisa; Bresnan, Eileen (2012). "Karenia: The biology and ecology of a toxic genus". Harmful Algae 14: 156–178. doi:10.1016/j.hal.2011.10.020. PMID 36733478.
- ↑ Buskey, E.J. (1995). "Growth and bioluminescence of Noctiluca scintillans on varying algal diets". Journal of Plankton Research 17 (1): 29–40. doi:10.1093/plankt/17.1.29.
- ↑ Thomson, Charles Wyville (2014) Voyage of the Challenger : The Atlantic Cambridge University Press, page235. ISBN:9781108074759.
- ↑ Grethe R. Hasle; Erik E. Syvertsen; Karen A. Steidinger; Karl Tangen (1996-01-25). "Marine Diatoms". in Carmelo R. Tomas. Identifying Marine Diatoms and Dinoflagellates. Academic Press. pp. 5–385. ISBN 978-0-08-053441-1. https://books.google.com/books?id=KQxPtwonlqoC. Retrieved 2013-11-13.
- ↑ Ald, S.M. (2007). "Diversity, Nomenclature, and Taxonomy of Protists". Syst. Biol. 56 (4): 684–689. doi:10.1080/10635150701494127. PMID 17661235.
- ↑ Moheimani, N.R.; Webb, J.P.; Borowitzka, M.A. (2012), "Bioremediation and other potential applications of coccolithophorid algae: A review. . Bioremediation and other potential applications of coccolithophorid algae: A review", Algal Research 1 (2): 120–133, doi:10.1016/j.algal.2012.06.002
- ↑ Taylor, A.R.; Chrachri, A.; Wheeler, G.; Goddard, H.; Brownlee, C. (2011). "A voltage-gated H+ channel underlying pH homeostasis in calcifying coccolithophores". PLOS Biology 9 (6): e1001085. doi:10.1371/journal.pbio.1001085. PMID 21713028.
- ↑ Wierer, U.; Arrighi, S.; Bertola, S.; Kaufmann, G.; Baumgarten, B.; Pedrotti, A.; Pernter, P.; Pelegrin, J. (2018). "The Iceman's lithic toolkit: Raw material, technology, typology and use". PLOS ONE 13 (6): e0198292. doi:10.1371/journal.pone.0198292. PMID 29924811. Bibcode: 2018PLoSO..1398292W.
- ↑ 225.0 225.1 Haq B.U. and Boersma A. (Eds.) (1998) Introduction to Marine Micropaleontology Elsevier. ISBN:9780080534961
- ↑ Bruckner, Monica (2020) "Paleoclimatology: How Can We Infer Past Climates?" SERC, Carleton College. Modified 23 July 2020. Retrieved 10 September 2020.
- ↑ Earth barreling toward 'Hothouse' state not seen in 50 million years, epic new climate record shows LiveScience, 10 September 2020.
- ↑ Westerhold, Thomas; Marwan, Norbert; Drury, Anna Joy; Liebrand, Diederik; Agnini, Claudia; Anagnostou, Eleni; Barnet, James S. K.; Bohaty, Steven M. et al. (11 September 2020). "An astronomically dated record of Earth's climate and its predictability over the last 66 million years". Science 369 (6509): 1383–1387. doi:10.1126/science.aba6853. PMID 32913105. Bibcode: 2020Sci...369.1383W. https://www.science.org/doi/10.1126/science.aba6853.
- ↑ Bob Yirka 29 Aug 2013
- ↑ Sussman: Oldest Plants, The Guardian , 2 May 2010
- ↑ "It's Okay to be Smart • the oldest living thing in the world: These". https://www.itsokaytobesmart.com/post/91481365622/siberian-actinobacteria-oldest-living-thing.
- ↑ Willerslev, Eske; Froese, Duane; Gilichinsky, David; Rønn, Regin; Bunce, Michael; Zuber, Maria T.; Gilbert, M. Thomas P.; Brand, Tina et al. (4 September 2007). "Ancient bacteria show evidence of DNA repair". Proceedings of the National Academy of Sciences 104 (36): 14401–14405. doi:10.1073/pnas.0706787104. PMID 17728401. Bibcode: 2007PNAS..10414401J.
- ↑ Egan, S., Fukatsu, T. and Francino, M.P., 2020. Opportunities and Challenges to Microbial Symbiosis Research in the Microbiome Era. Frontiers in Microbiology, 11. doi:10.3389/fmicb.2020.01150. 50px Modified text was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
- ↑ Margulis, Lynn; Fester, René (1991). Symbiosis as a Source of Evolutionary Innovation. MIT Press. ISBN 9780262132695. https://mitpress.mit.edu/books/symbiosis-source-evolutionary-innovation.
- ↑ Bordenstein, Seth R.; Theis, Kevin R. (2015). "Host Biology in Light of the Microbiome: Ten Principles of Holobionts and Hologenomes". PLOS Biol 13 (8): e1002226. doi:10.1371/journal.pbio.1002226. ISSN 1545-7885. PMID 26284777.
- ↑ Theis, K.R., Dheilly, N.M., Klassen, J.L., Brucker, R.M., Baines, J.F., Bosch, T.C., Cryan, J.F., Gilbert, S.F., Goodnight, C.J., Lloyd, E.A. and Sapp, J. (2016) "Getting the hologenome concept right: an eco-evolutionary framework for hosts and their microbiomes". Msystems, 1(2). doi:10.1128/mSystems.00028-16.
 Modified text was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
Modified text was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
- ↑ Bulgarelli, D., Schlaeppi, K., Spaepen, S., Van Themaat, E.V.L. and Schulze-Lefert, P. (2013) "Structure and functions of the bacterial microbiota of plants". Annual review of plant biology, 64: 807–838. doi:10.1146/annurev-arplant-050312-120106.
- ↑ Vandenkoornhuyse, Philippe; Quaiser, Achim; Duhamel, Marie; Le Van, Amandine; Dufresne, Alexis (2015-06-01). "The importance of the microbiome of the plant holobiont". The New Phytologist 206 (4): 1196–1206. doi:10.1111/nph.13312. ISSN 1469-8137. PMID 25655016.
- ↑ Sánchez-Cañizares, C., Jorrín, B., Poole, P.S. and Tkacz, A. (2017) "Understanding the holobiont: the interdependence of plants and their microbiome". Current Opinion in Microbiology, 38: 188–196. doi:10.1016/j.mib.2017.07.001.
- ↑ Douglas, A.E. and Werren, J.H. (2016) "Holes in the hologenome: why host-microbe symbioses are not holobionts". MBio, 7(2). doi:10.1128/mBio.02099-15.
- ↑ Thompson, J.R., Rivera, H.E., Closek, C.J. and Medina, M. (2015) "Microbes in the coral holobiont: partners through evolution, development, and ecological interactions". Frontiers in cellular and infection microbiology, 4: 176. doi:10.3389/fcimb.2014.00176.
- ↑ Pita, L., Rix, L., Slaby, B.M., Franke, A. and Hentschel, U. (2018) "The sponge holobiont in a changing ocean: from microbes to ecosystems". Microbiome, 6(1): 46. doi:10.1186/s40168-018-0428-1.
 Modified text was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
Modified text was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
- ↑ Ugarelli, K., Chakrabarti, S., Laas, P. and Stingl, U. (2017) "The seagrass holobiont and its microbiome". Microorganisms, 5(4): 81. doi:10.3390/microorganisms5040081.
 Modified text was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
Modified text was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
- ↑ Cavalcanti, G.S., Shukla, P., Morris, M., Ribeiro, B., Foley, M., Doane, M.P., Thompson, C.C., Edwards, M.S., Dinsdale, E.A. and Thompson, F.L. (2018) "Rhodoliths holobionts in a changing ocean: host-microbes interactions mediate coralline algae resilience under ocean acidification". BMC Genomics, 19(1): 1–13. doi:10.1186/s12864-018-5064-4.
 Modified text was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
Modified text was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
- ↑ Knowlton, N. and Rohwer, F. (2003) "Multispecies microbial mutualisms on coral reefs: the host as a habitat". The American Naturalist, 162(S4): S51-S62. doi:10.1086/378684.
- ↑ Pollock, F. Joseph; McMinds, Ryan; Smith, Styles; Bourne, David G.; Willis, Bette L.; Medina, Mónica; Thurber, Rebecca Vega; Zaneveld, Jesse R. (2018-11-22). "Coral-associated bacteria demonstrate phylosymbiosis and cophylogeny" (in en). Nature Communications 9 (1): 4921. doi:10.1038/s41467-018-07275-x. ISSN 2041-1723. PMID 30467310. Bibcode: 2018NatCo...9.4921P.
- ↑ Wilhelm, Steven W.; Suttle, Curtis A. (1999). "Viruses and Nutrient Cycles in the Sea". BioScience 49 (10): 781–788. doi:10.2307/1313569. ISSN 1525-3244.
- ↑ Weinbauer, Markus G. (2007). "Synergistic and antagonistic effects of viral lysis and protistan grazing on bacterial biomass, production and diversity". Environmental Microbiology 9 (3): 777–788. doi:10.1111/j.1462-2920.2006.01200.x. PMID 17298376. Bibcode: 2007EnvMi...9..777W.
- ↑ Robinson, Carol, and Nagappa Ramaiah. "Microbial heterotrophic metabolic rates constrain the microbial carbon pump." The American Association for the Advancement of Science, 2011.
- ↑ Gutierrez, MH; Jara, AM; Pantoja, S (2016). "Fungal parasites infect marine diatoms in the upwelling ecosystem of the Humboldt current system off central Chile". Environ Microbiol 18 (5): 1646–1653. doi:10.1111/1462-2920.13257. PMID 26914416. Bibcode: 2016EnvMi..18.1646G.
- ↑ What is marine snow? NOAA National Ocean Service. Updated:06/25/18.
- ↑ Monroy, P., Hernández-García, E., Rossi, V. and López, C. (2017) "Modeling the dynamical sinking of biogenic particles in oceanic flow". Nonlinear Processes in Geophysics, 24(2): 293–305. doi:10.5194/npg-24-293-2017. 50px Modified text was copied from this source, which is available under a Creative Commons Attribution 3.0 International License.
- ↑ Simon, M., Grossart, H., Schweitzer, B. and Ploug, H. (2002) "Microbial ecology of organic aggregates in aquatic ecosystems". Aquatic microbial ecology, 28: 175–211. doi:10.3354/ame028175.
- ↑ Boetius, Antje; Anesio, Alexandre M.; Deming, Jody W.; Mikucki, Jill A.; Rapp, Josephine Z. (1 November 2015). "Microbial ecology of the cryosphere: sea ice and glacial habitats". Nature Reviews Microbiology 13 (11): 677–690. doi:10.1038/nrmicro3522. PMID 26344407.
- ↑ Lutz, Richard A.; Kennish, Michael J. (1993). "Ecology of deep-sea hydrothermal vent communities: A review" (in en). Reviews of Geophysics 31 (3): 211. doi:10.1029/93rg01280. ISSN 8755-1209. Bibcode: 1993RvGeo..31..211L.
- ↑ Kádár, Enikõ; Costa, Valentina; Santos, Ricardo S.; Powell, Jonathan J. (July 2006). "Tissue partitioning of micro-essential metals in the vent bivalve Bathymodiolus azoricus and associated organisms (endosymbiont bacteria and a parasite polychaete) from geochemically distinct vents of the Mid-Atlantic Ridge". Journal of Sea Research 56 (1): 45–52. doi:10.1016/j.seares.2006.01.002. ISSN 1385-1101. Bibcode: 2006JSR....56...45K.
- ↑ Anderson, Rika E.; Brazelton, William J.; Baross, John A. (2011). "Is the Genetic Landscape of the Deep Subsurface Biosphere Affected by Viruses?". Frontiers in Microbiology 2: 219. doi:10.3389/fmicb.2011.00219. PMID 22084639.
- ↑ Anderson, Rika E.; Brazelton, William J.; Baross, John A. (2011). "Is the genetic landscape of the deep subsurface biosphere affected by viruses?". Frontiers in Microbiology 2: 219. doi:10.3389/fmicb.2011.00219. ISSN 1664-302X. PMID 22084639.
- ↑ Suttle, Curtis A. (September 2005). "Viruses in the sea". Nature 437 (7057): 356–361. doi:10.1038/nature04160. ISSN 0028-0836. PMID 16163346. Bibcode: 2005Natur.437..356S.
- ↑ Suttle, Curtis A. (October 2007). "Marine viruses — major players in the global ecosystem" (in En). Nature Reviews Microbiology 5 (10): 801–812. doi:10.1038/nrmicro1750. ISSN 1740-1526. PMID 17853907.
- ↑ Anderson, Rika E.; Sogin, Mitchell L.; Baross, John A. (2014-10-03). "Evolutionary Strategies of Viruses, Bacteria and Archaea in Hydrothermal Vent Ecosystems Revealed through Metagenomics". PLOS ONE 9 (10): e109696. doi:10.1371/journal.pone.0109696. ISSN 1932-6203. PMID 25279954. Bibcode: 2014PLoSO...9j9696A.
- ↑ Ortmann, Alice C.; Suttle, Curtis A. (August 2005). "High abundances of viruses in a deep-sea hydrothermal vent system indicates viral mediated microbial mortality". Deep Sea Research Part I: Oceanographic Research Papers 52 (8): 1515–1527. doi:10.1016/j.dsr.2005.04.002. ISSN 0967-0637. Bibcode: 2005DSRI...52.1515O.
- ↑ Breitbart, Mya (2012-01-15). "Marine Viruses: Truth or Dare" (in en). Annual Review of Marine Science 4 (1): 425–448. doi:10.1146/annurev-marine-120709-142805. ISSN 1941-1405. PMID 22457982. Bibcode: 2012ARMS....4..425B.
- ↑ Goldenfeld, Nigel; Woese, Carl (January 2007). "Biology's next revolution". Nature 445 (7126): 369. doi:10.1038/445369a. ISSN 0028-0836. PMID 17251963. Bibcode: 2007Natur.445..369G.
- ↑ Deep Carbon Observatory (10 December 2018). "Life in deep Earth totals 15 to 23 billion tons of carbon – hundreds of times more than humans". EurekAlert!. https://www.eurekalert.org/pub_releases/2018-12/tca-lid120318.php.
- ↑ Dockrill, Peter (11 December 2018). "Scientists Reveal a Massive Biosphere of Life Hidden Under Earth's Surface". Science Alert. https://www.sciencealert.com/scientists-lift-lid-on-massive-biosphere-of-life-hidden-under-earth-s-surface.
- ↑ Gabbatiss, Josh (11 December 2018). "Massive 'deep life' study reveals billions of tonnes of microbes living far beneath Earth's surface". The Independent. https://www.independent.co.uk/news/science/deep-life-microbes-underground-bacteria-earth-surface-carbon-observatory-science-study-a8677521.html.
- ↑ Lollar, Garnet S.; Warr, Oliver; Telling, Jon; Osburn, Magdalena R.; Sherwood Lollar, Barbara (2019). "'Follow the Water': Hydrogeochemical Constraints on Microbial Investigations 2.4 km Below Surface at the Kidd Creek Deep Fluid and Deep Life Observatory". Geomicrobiology Journal 36 (10): 859–872. doi:10.1080/01490451.2019.1641770. Bibcode: 2019GmbJ...36..859L.
- ↑ World's Oldest Groundwater Supports Life Through Water-Rock Chemistry , 29 July 2019, deepcarbon.net.
- ↑ Strange life-forms found deep in a mine point to vast 'underground Galapagos', By Corey S. Powell, 7 Sept. 2019, nbcnews.com.
- ↑ Oldest Water on Earth Found Deep Within the Canadian Shield, 14 December 2016, Maggie Romuld
- ↑ Wu, Katherine J. (28 July 2020). "These Microbes May Have Survived 100 Million Years Beneath the Seafloor – Rescued from their cold, cramped and nutrient-poor homes, the bacteria awoke in the lab and grew.". The New York Times. https://www.nytimes.com/2020/07/28/science/microbes-100-million-years-old.html.
- ↑ Morono, Yuki (28 July 2020). "Aerobic microbial life persists in oxic marine sediment as old as 101.5 million years". Nature Communications 11 (3626): 3626. doi:10.1038/s41467-020-17330-1. PMID 32724059. Bibcode: 2020NatCo..11.3626M.
- ↑ 274.0 274.1 Filee, J.; Tetart, F.; Suttle, C. A.; Krisch, H. M. (2005). "Marine T4-type bacteriophages, a ubiquitous component of the dark matter of the biosphere". Proceedings of the National Academy of Sciences 102 (35): 12471–12476. doi:10.1073/pnas.0503404102. ISSN 0027-8424. PMID 16116082. Bibcode: 2005PNAS..10212471F.
- ↑ University of Tennessee at Knoxville (25 September 2018). "Study: Microbial dark matter dominates Earth's environments". Eurekalert!. https://www.eurekalert.org/pub_releases/2018-09/uota-smd092518.php.
- ↑ Hedlund, Brian P.; Dodsworth, Jeremy A.; Murugapiran, Senthil K.; Rinke, Christian; Woyke, Tanja (2014). "Impact of single-cell genomics and metagenomics on the emerging view of extremophile "microbial dark matter"". Extremophiles 18 (5): 865–875. doi:10.1007/s00792-014-0664-7. ISSN 1431-0651. PMID 25113821.
- ↑ May, R.M. (1988). "How many species are there on earth?". Science 241 (4872): 1441–1449. doi:10.1126/science.241.4872.1441. PMID 17790039. Bibcode: 1988Sci...241.1441M.
- ↑ Locey, K.J.; Lennon, J.T. (2016). "Scaling laws predict global microbial diversity". Proceedings of the National Academy of Sciences 113 (21): 5970–5975. doi:10.1073/pnas.1521291113. PMID 27140646. Bibcode: 2016PNAS..113.5970L.
- ↑ 279.0 279.1 Vitorino, L.C.; Bessa, L.A. (2018). "Microbial diversity: the gap between the estimated and the known". Diversity 10 (2): 46. doi:10.3390/d10020046. 50px Modified text was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
- ↑ 280.0 280.1 280.2 Karsenti, E.; Acinas, S.G.; Bork, P.; Bowler, C.; De Vargas, C.; Raes, J.; Sullivan, M.; Arendt, D. et al. (2011). "A holistic approach to marine eco-systems biology". PLOS Biology 9 (10): e1001177. doi:10.1371/journal.pbio.1001177. PMID 22028628.
- ↑ "The winds of (evolutionary) change: breathing new life into microbiology". Journal of Bacteriology 176 (1): 1–6. 1994. doi:10.2172/205047. PMID 8282683.
- ↑ Woese, CR (1987). "Bacterial evolution". Microbiological Reviews 51 (2): 221–71. doi:10.1128/MMBR.51.2.221-271.1987. PMID 2439888.
- ↑ 283.0 283.1 Collins, R. Eric (2019) The Small and Mighty – Microbial Life NOAA: Ocean Exploration and Research, Gulf of Alaska Seamounts 2019 Expedition.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ Käse L, Geuer JK. (2018) "Phytoplankton Responses to Marine Climate Change – An Introduction". In Jungblut S., Liebich V., Bode M. (Eds) YOUMARES 8–Oceans Across Boundaries: Learning from each other, pages 55–72, Springer. doi:10.1007/978-3-319-93284-2_5.
 Modified text was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
Modified text was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
- ↑ Franco-Duarte, R.; Černáková, L.; Kadam, S.; Kaushik, K.; Salehi, B.; Bevilacqua, A.; Corbo, M.R.; Antolak, H. et al. (2019). "Advances in Chemical and Biological Methods to Identify Microorganisms—From Past to Present". Microorganisms 7 (5): 130. doi:10.3390/microorganisms7050130. PMID 31086084.
 Modified text was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
Modified text was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
- ↑ Lozupone, C.A., Stombaugh, J.I., Gordon, J.I., Jansson, J.K. and Knight, R. (2012) "Diversity, stability and resilience of the human gut microbiota". Nature, 489(7415): 220–230. doi:10.1038/nature11550.
- ↑ Venter, J.C., Remington, K., Heidelberg, J.F., Halpern, A.L., Rusch, D., Eisen, J.A., Wu, D., Paulsen, I., Nelson, K.E., Nelson, W. and Fouts, D.E. (2004) "Environmental genome shotgun sequencing of the Sargasso Sea". Science, 304(5667): 66–74. doi:10.1126/science.1093857.
- ↑ Liu, L., Li, Y., Li, S., Hu, N., He, Y., Pong, R., Lin, D., Lu, L. and Law, M. (2012) "Comparison of next-generation sequencing systems". BioMed Research International, 2012: 251364. doi:10.1155/2012/251364.
- ↑ Berg, Gabriele; Daria Rybakova, Doreen Fischer, Tomislav Cernava, Marie-Christine Champomier Vergès, Trevor Charles, Xiaoyulong Chen, Luca Cocolin, Kellye Eversole, Gema Herrero Corral, Maria Kazou, Linda Kinkel, Lene Lange, Nelson Lima, Alexander Loy, James A. Macklin, Emmanuelle Maguin, Tim Mauchline, Ryan McClure, Birgit Mitter, Matthew Ryan, Inga Sarand, Hauke Smidt, Bettina Schelkle, Hugo Roume, G. Seghal Kiran, Joseph Selvin, Rafael Soares Correa de Souza, Leo van Overbeek, Brajesh K. Singh, Michael Wagner, Aaron Walsh, Angela Sessitsch and Michael Schloter (2020) "Microbiome definition re-visited: old concepts and new challenges". Microbiome, 8(103): 1–22. doi:10.1186/s40168-020-00875-0.
 Modified text was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
Modified text was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
- ↑ Kodzius, R.; Gojobori, T. (2015). "Marine metagenomics as a source for bioprospecting". Marine Genomics 24: 21–30. doi:10.1016/j.margen.2015.07.001. PMID 26204808. Bibcode: 2015MarGn..24...21K. 50px Modified text was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
- ↑ Giuliano, Laura; Barbier, Michele; Briand, Frederic (Eds.) (2010). Marine Omics. Microbial Biotechnology special issue 3, n°5: Wiley- Blackwell. pp. 489–621.[1]
- ↑ Holtorf, Hauke; Guitton, Marie-Christine; Reski, Ralf (2002). "Plant functional genomics". Naturwissenschaften 89 (6): 235–249. doi:10.1007/s00114-002-0321-3. PMID 12146788. Bibcode: 2002NW.....89..235H.
- ↑ Cain, A.K., Barquist, L., Goodman, A.L., Paulsen, I.T., Parkhill, J. and van Opijnen, T. (2020) "A decade of advances in transposon-insertion sequencing". Nature Reviews Genetics, 21: 526–540. doi:10.1038/s41576-020-0244-x.
- ↑ Zhang, W., Li, F. and Nie, L. (2010) "Integrating multiple 'omics' analysis for microbial biology: application and methodologies". Microbiology, 156(2): 287–301. doi:10.1099/mic.0.034793-0.
- ↑ Singh, R.P. and Kothari, R. (2017) "The omics era and host microbiomes". In: Singh RP, Kothari R, Koringa PG and Singh SP (Eds.) Understanding Host-Microbiome Interactions – An Omics Approach, pages 3–12, Springer. ISBN:9789811050503. doi:10.1007/978-981-10-5050-3_1
- ↑ Capotondi, A., Jacox, M., Bowler, C., Kavanaugh, M., Lehodey, P., Barrie, D., Brodie, S., Chaffron, S., Cheng, W., Dias, D.F., Eveillard, D. and 20 others (2019) "Observational needs supporting marine ecosystems modeling and forecasting: from the global ocean to regional and coastal systems". Frontiers in Marine Science, 6: 623. doi:10.3389/fmars.2019.00623. 50px Modified text was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
- ↑ Sunagawa, S., Coelho, L. P., Chaffron, S., Kultima, J. R., Labadie, K., Salazar, G., et al. (2015). "Ocean plankton. Structure and function of the global ocean microbiome". Science, 348: 1261359. doi:10.1126/science.1261359.
- ↑ Guidi, L., Chaffron, S., Bittner, L., Eveillard, D., Larhlimi, A., Roux, S., et al. (2016). "Plankton networks driving carbon export in the oligotrophic ocean". Nature, 532: 465–470. doi:10.1038/nature16942.
- ↑ Guyennon, A., Baklouti, M., Diaz, F., Palmieri, J., Beuvier, J., Lebaupin-Brossier, C., et al. (2015). "New insights into the organic carbon export in the Mediterranean Sea from 3-D modelling". Biogeosciences, 11: 425–442. doi:10.5194/bg-12-7025-2015.
- ↑ Louca, S., Parfrey, L. W., and Doebeli, M. (2016). "Decoupling function and taxonomy in the global ocean microbiome". Science, 353: 1272–1277. doi:10.1126/science.aaf4507.
- ↑ Coles, V. J., Stukel, M. R., Brooks, M. T., Burd, A., Crump, B. C., Moran, M. A., et al. (2017). "Ocean biogeochemistry modeled with emergent trait-based genomics". Science, 358: 1149–1154. doi:10.1126/science.aan5712.
- ↑ Mock, T., Daines, S.J., Geider, R., Collins, S., Metodiev, M., Millar, A.J., Moulton, V. and Lenton, T.M. (2016) "Bridging the gap between omics and earth system science to better understand how environmental change impacts marine microbes". Global Change Biology, 22(1): 61–75. doi:10.1111/gcb.12983. 50px Modified text was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
- ↑ 303.0 303.1 Toseland A, Daines SJ and Clark JR et al. (2013) "The impact of temperature on marine phytoplankton resource allocation and metabolism". Nature Climate Change, 3: 979– 984. doi:10.1038/nclimate1989.
- ↑ Martiny AC, Pham CTA, Primeau FW, Vrugt JA, Moore JK, Levin SA and Lomas MW (2013) "Strong latitudinal patterns in the elemental ratios of marine plankton and organic matter". Nature Geoscience, 6: 279–283. doi:10.1038/ngeo1757.
General references
- Haq B.U. and Boersma A. (Eds.) (1998) Introduction to Marine Micropaleontology Elsevier. ISBN:9780080534961
- Kirchman, David L. (2018) Processes in Microbial Ecology Oxford University Press. ISBN:9780192506474
- Helmreich, Stefan (2009) Alien Ocean: Anthropological Voyages in Microbial Seas University of California Press. ISBN:9780520942608
- Munn, Colin B. (2019) Marine Microbiology: Ecology & Applications CRC Press. ISBN:9780429592362
- Stal LJ and Cretoiu MS (2016) The Marine Microbiome: An Untapped Source of Biodiversity and Biotechnological Potential Springer. ISBN:9783319330006
- Middelboe M and Corina Brussaard CPD (Eds.) Marine Viruses 2016, MDPI. ISBN:978-3-03842-621-9 Download PDF
- Ohtsuka S, Suzaki T, Horiguchi T, Suzuki N and Not F (Eds.) (2015) Marine Protists: Diversity and Dynamics Springer. ISBN:9784431551300
- Johnson MD and V. Moeller HV (2019) Mixotrophy in Protists: From Model Systems to Mathematical Models, 2nd Edition, Frontiers Media. ISBN:9782889631483
- Radiolarian art
 |
 KSF
KSF