Cation-anion radius ratio
 From HandWiki - Reading time: 3 min
From HandWiki - Reading time: 3 min
In condensed matter physics and inorganic chemistry, the cation-anion radius ratio can be used to predict the crystal structure of an ionic compound based on the relative size of its atoms. It is defined as the ratio of the ionic radius of the positively charged cation to the ionic radius of the negatively charged anion in a cation-anion compound. Anions are larger than cations. Large sized anions occupy lattice sites, while small sized cations are found in voids. In a given structure, the ratio of cation radius to anion radius is called the radius ratio. This is simply given by .
Ratio rule and stability

The radius ratio rule defines a critical radius ratio for different crystal structures, based on their coordination geometry.[1] The idea is that the anions and cations can be treated as incompressible spheres, meaning the crystal structure can be seen as a kind of unequal sphere packing. The allowed size of the cation for a given structure is determined by the critical radius ratio.[2] If the cation is too small, then it will attract the anions into each other and they will collide hence the compound will be unstable due to anion-anion repulsion; this occurs when the radius ratio drops below the critical radius ratio for that particular structure. At the stability limit the cation is touching all the anions and the anions are just touching at their edges. For radius ratios greater than the critical ratius ratio, the structure is expected to be stable.
The rule is not obeyed for all compounds. By one estimate, the crystal structure can only be guessed about 2/3 of the time.[3] Errors in prediction are partly due to the fact that real chemical compounds are not purely ionic, they display some covalent character.[1]
The table below gives the relation between critical radius ratio, , and coordination number, , which may be obtained from a simple geometrical proof.[4]
| Critical Radius Ratio | Coordination number | Type of void | Crystal structure | Examples |
|---|---|---|---|---|
| 0.1547 | 3 | 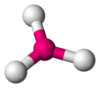 Triangular Planar |
frameless α-B 2O 3 structure |
B2O3 |
| 0.2247 | 4 | 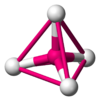 Tetrahedral |
frameless Zincblende structure |
ZnS, CuCl |
| 0.4142 | 6 | 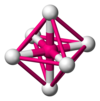 Octahedral |
frameless Rock salt structure |
NaCl, MgO |
| 0.7320 | 8 | 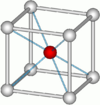 Cubic |
frameless CsCl structure |
CsCl, NH4Br |
History
The radius ratio rule was first proposed by Gustav F. Hüttig in 1920.[5][6] In 1926, Victor Goldschmidt[5] extended the use to ionic lattices.[7][8][9] In 1929, the rule was incorporated as the first of Pauling's rules for crystal structures.[10]
See also
References
- ↑ 1.0 1.1 Michmerhuizen, Anna; Rose, Karine; Annankra, Wentiirim; Vander Griend, Douglas A. (2017-08-09). "Radius Ratio Rule Rescue". Journal of Chemical Education (American Chemical Society (ACS)) 94 (10): 1480–1485. doi:10.1021/acs.jchemed.6b00970. ISSN 0021-9584.
- ↑ Pauling, Linus (1960). Nature of the Chemical Bond (3rd ed.). Ithaca, New York: Cornell University Press. p. 544. ISBN 9780801403330. https://books.google.com/books?id=L-1K9HmKmUUC&pg=PA544.
- ↑ Nathan, Lawrence C. (1985). "Predictions of crystal structure based on radius ratio: How reliable are they?". Journal of Chemical Education (American Chemical Society (ACS)) 62 (3): 215. doi:10.1021/ed062p215. ISSN 0021-9584.
- ↑ Toofan, Jahansooz (1994). "A Simple Expression between Critical Radius Ratio and Coordination Number". Journal of Chemical Education (American Chemical Society (ACS)) 71 (2): 147. doi:10.1021/ed071p147. ISSN 0021-9584. (and Erratum 71(9): 749 doi:10.1021/ed071p749), Following the erratum, equations should read and .
- ↑ 5.0 5.1 Jensen, William B. (2010-04-23). "The Origin of the Ionic-Radius Ratio Rules". Journal of Chemical Education (American Chemical Society (ACS)) 87 (6): 587–588. doi:10.1021/ed100258f. ISSN 0021-9584.
- ↑ Hüttig, Gustav F. (1920-11-11). "Notiz zur Geometrie der Koordinationszahl" (in de). Zeitschrift für anorganische und allgemeine Chemie (Wiley) 114 (1): 24–26. doi:10.1002/zaac.19201140103. ISSN 0863-1786. https://zenodo.org/record/1428172.
- ↑ Goldschmidt, V.; Barth, T.; Lunde, G.; Zachariasen, W. (1926) (in de). Geochemische Verteilungsgesetze der Elemente. VII. Die Gesetze der Krystallochemie. Oslo: Dybwad. pp. 112–117. OCLC 174577644. https://publikationen.ub.uni-frankfurt.de/frontdoor/index/index/docId/19275.
- ↑ Goldschmidt, V. (1927) (in de). Geochemische Verteilungsgesetze der Elemente. VIII. Untersuchungen über Bau und Eigenschaften von Krystallen. Oslo: Dybwad. pp. 14–17. OCLC 19831825.
- ↑ Goldschmidt, V. M. (1929). "Crystal structure and chemical constitution". Transactions of the Faraday Society (Royal Society of Chemistry (RSC)) 25: 253. doi:10.1039/tf9292500253. ISSN 0014-7672.
- ↑ Pauling, Linus (1929). "The principles determining the structure of complex ionic crystals". Journal of the American Chemical Society (American Chemical Society (ACS)) 51 (4): 1010–1026. doi:10.1021/ja01379a006. ISSN 0002-7863.
 |
 KSF
KSF