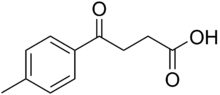
4-(4-Methylphenyl)-4-oxobutanoic acid
Topic: Chemistry
 From HandWiki - Reading time: 2 min
From HandWiki - Reading time: 2 min
| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
4-(4-Methylphenyl)-4-oxobutanoic acid | |
| Other names
3-(4-Methylbenzoyl)propionic acid
3-(p-Toluoyl)propionic acid 4-(4-Methylphenyl)-4-oxobutyric acid | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C11H12O3 | |
| Molar mass | 192.214 g·mol−1 |
| Appearance | White powder |
| Melting point | 129 °C (264 °F; 402 K) |
| Insoluble | |
| Hazards[1] | |
| Main hazards | Flammable |
| GHS pictograms | 
|
| GHS Signal word | Warning |
| H315, H319, H335 | |
| P261, P264, P271, P280, P302+352, P304+340, P305+351+338, P312, P332+313, P337+313, P362, P403+233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
4-(4-Methylphenyl)-4-oxobutanoic acid is an organic carboxylic acid. The preparation of it is used for undergraduate teaching of organic chemistry synthesis.
Preparation
4-(4-Methylphenyl)-4-oxobutanoic acid can be prepared by a Friedel–Crafts reaction between toluene and succinic anhydride catalyzed by a Lewis acid such as aluminium chloride.[2]
References
- ↑ "3-(4-Methylbenzoyl)propionic acid" (in en). https://pubchem.ncbi.nlm.nih.gov/compound/244162#section=Safety-and-Hazards.
- ↑ Preparation of 4-(p-methylphenyl)-4-oxobutanoic acid, Li Cuijuan, Lv Mingquan, Han Shuying, Xu Tanfeng (College of Chemistry and Molecule Engineering, Peking University), College Chemistry, Vol 20. Period 2.
 |
Licensed under CC BY-SA 3.0 | Source: https://handwiki.org/wiki/Chemistry:4-(4-Methylphenyl)-4-oxobutanoic_acid1 | ↧ Download this article as ZWI file
 KSF
KSF