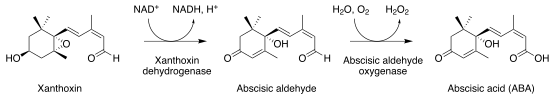
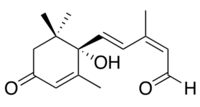
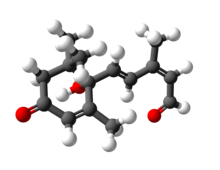
Abscisic aldehyde
Topic: Chemistry
 From HandWiki - Reading time: 2 min
From HandWiki - Reading time: 2 min
| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
(2Z,4E)-5-[(1S)-1-Hydroxy-2,6,6-trimethyl-4-oxocyclohex-2-en-1-yl]-3-methylpenta-2,4-dienal | |
| Other names
Abscisyl aldehyde
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C15H20O3 | |
| Molar mass | 248.322 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Abscisic aldehyde is an intermediate in the biosynthesis of the plant hormone abscisic acid.[1][2] It is produced by the dehydrogenation of xanthoxin by xanthoxin dehydrogenases, which is an NAD+ dependent short-chain dehydrogenase,[3] followed by selective oxidation by abscisic aldehyde oxygenase.[4]
References
- ↑ Ram K. Sindhu, David H. Griffin and Daniel C. Walton (1990). "Abscisic Aldehyde Is an Intermediate in the Enzymatic Conversion of Xanthoxin to Abscisic Acid in Phaseolus vulgaris L. Leaves". Plant Physiology 93 (2): 689–694. doi:10.1104/pp.93.2.689. PMID 16667524. PMC 1062571. http://www.plantphysiol.org/content/93/2/689.short.
- ↑ Seo, M; Koshiba, T (2002). "Complex regulation of ABA biosynthesis in plants". Trends in Plant Science 7 (1): 41–8. doi:10.1016/S1360-1385(01)02187-2. PMID 11804826.
- ↑ Gonzalez-Guzman, M. (25 July 2002). "The Short-Chain Alcohol Dehydrogenase ABA2 Catalyzes the Conversion of Xanthoxin to Abscisic Aldehyde". The Plant Cell Online 14 (8): 1833–1846. doi:10.1105/tpc.002477. PMID 12172025.
- ↑ Seo, Mitsunori; Koiwai, Hanae; Akaba, Shuichi; Komano, Teruya; Oritani, Takayuki; Kamiya, Yuji; Koshiba, Tomokazu (August 2000). "Abscisic aldehyde oxidase in leaves of Arabidopsis thaliana". The Plant Journal 23 (4): 481–488. doi:10.1046/j.1365-313x.2000.00812.x. PMID 10972874.
 |
Licensed under CC BY-SA 3.0 | Source: https://handwiki.org/wiki/Chemistry:Abscisic_aldehyde6 views | ↧ Download this article as ZWI file
 KSF
KSF