Alamandine
Topic: Chemistry
 From HandWiki - Reading time: 4 min
From HandWiki - Reading time: 4 min
| |
| Names | |
|---|---|
| IUPAC name
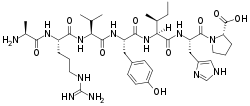
(2S)-1-[(2S)-2-[[(2S,3S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-aminopropanoyl]amino]-5-(diaminomethylideneamino)pentanoyl]amino]-3-methylbutanoyl]amino]-3-(4-hydroxyphenyl)propanoyl]amino]-3-methylpentanoyl]amino]-3-(1H-imidazol-5-yl)propanoyl]pyrrolidine-2-carboxylic acid
| |
| Other names
H-Ala-Arg-Val-Tyr-Ile-His-Pro-OH; L-alanyl-L-arginyl-L-valyl-L-tyrosyl-L-isoleucyl-L-histidyl-L-proline
| |
| Identifiers | |
3D model (JSmol)
|
|
| Abbreviations | ARVYIHP |
| ChEBI | |
| ChemSpider | |
| KEGG | |
PubChem CID
|
|
| |
| |
| Properties | |
| C40H62N12O9 | |
| Molar mass | 855 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Alamandine is a member of renin-angiotensin system (RAS) with cardiovascular functions that are protective and opposing to the classical axis. Alamandine is a product of ACE2-dependent catalytic hydrolysis of angiotensin A (Ang A) and can also be generated by decarboxylation of aspartic acid residue of Ang-(1-7).[1] Ang A is Ala1-Ang II (alanine in place of aspartic acid). In mononuclear leucocytes, angiotensin II (Ang II) is converted to Ang A by decarboxylation of aspartic acid. Ang A is detected in human circulation and was shown to be higher in individuals with end-stage renal disease.[1]
Receptor
Mice deficient of MrgD receptor showed myocardial pathology with dilated cardiomyopathy and a marked decrease in systolic function further supporting cardioprotective pharmacology of alamandine.
Vascular actions
Alamandine produced endothelium-dependent vasorelaxation that was blocked by D-Pro7-Ang-(1-7), an MrgD receptor antagonist but not by A779, an antagonist of Mas receptor.[2] In agreement with this, Tetzner et al [3] showed that alamandine activated cAMP formation in endothelial and mesangial cells transfected with MrgD. Importantly, oral administration of an alamandine/2-hydroxypropyl β-cyclodextrin (HPβCD) complex produced an antihypertensive effect in spontaneously hypertensive rats (SHR).[2] In the same model, vascular remodeling in the ascending aorta was also prevented that was associated with decreased pro-inflammatory (IL-1β, TNF alpha and CCL2) and pro-fibrotic factors (MMP2 and TGF β1), and increased pro-resolution markers (MRC1 and FIZZ1)[4]
Cardiac actions
Almandine showed cardioprotective effects in experimental models of pressure overload. In mice undergoing transverse aortic constriction, Alamandine prevented cardiac hypertrophy and fibrosis that was shown to be mediated partly via decreased ERK1/2 phosphorylation, TGF β1 and MMP2, and increased AMPKα phosphorylation.[5]
Central effects
Experimental evidence was provided for an important role of this peptide in the central regulation of blood pressure. A study by Marins et al[6] showed evidence for central regulation of hemodynamics specifically by acting on MrgD receptors in rostral insular cortex. Microinjection of alamandine in this area elevated mean arterial blood pressure and renal sympathetic activity that were blocked by D-Pro7-Ang-(1-7), an MrgD antagonist.
References
- ↑ 1.0 1.1 Jankowski, Vera; Vanholder, Raymond; van der Giet, Markus; Tölle, Markus; Karadogan, Sevil; Gobom, Johan; Furkert, Jens; Oksche, Alexander et al. (February 2007). "Mass-spectrometric identification of a novel angiotensin peptide in human plasma". Arteriosclerosis, Thrombosis, and Vascular Biology 27 (2): 297–302. doi:10.1161/01.ATV.0000253889.09765.5f. PMID 17138938.
- ↑ 2.0 2.1 Lautner, Roberto Queiroga; Villela, Daniel C.; Fraga-Silva, Rodrigo A.; Silva, Neiva; Verano-Braga, Thiago; Costa-Fraga, Fabiana; Jankowski, Joachim; Jankowski, Vera et al. (12 April 2013). "Discovery and characterization of alamandine: a novel component of the renin-angiotensin system". Circulation Research 112 (8): 1104–1111. doi:10.1161/CIRCRESAHA.113.301077. PMID 23446738.
- ↑ Tetzner, Anja; Naughton, Maura; Gebolys, Kinga; Eichhorst, Jenny; Sala, Esther; Villacañas, Óscar; Walther, Thomas (15 August 2018). "Decarboxylation of Ang-(1-7) to Ala1-Ang-(1-7) leads to significant changes in pharmacodynamics". European Journal of Pharmacology 833: 116–123. doi:10.1016/j.ejphar.2018.05.031. PMID 29792841. https://pubmed.ncbi.nlm.nih.gov/29792841/. Retrieved 28 May 2022.
- ↑ de Souza-Neto, Fernando Pedro; Silva, Mario de Morais E.; Santuchi, Melissa de Carvalho; de Alcântara-Leonídio, Thaís Cristina; Motta-Santos, Daisy; Oliveira, Aline Cristina; Melo, Marcos Barrouin; Canta, Giovanni Naves et al. (15 March 2019). "Alamandine attenuates arterial remodelling induced by transverse aortic constriction in mice". Clinical Science 133 (5): 629–643. doi:10.1042/CS20180547. PMID 30737255. https://pubmed.ncbi.nlm.nih.gov/30737255/. Retrieved 28 May 2022.
- ↑ Silva, Mário Morais; de Souza-Neto, Fernando Pedro; Jesus, Itamar Couto Guedes de; Gonçalves, Gleisy Kelly; Santuchi, Melissa de Carvalho; Sanches, Bruno de Lima; de Alcântara-Leonídio, Thaís Cristina; Melo, Marcos Barrouin et al. (1 January 2021). "Alamandine improves cardiac remodeling induced by transverse aortic constriction in mice". American Journal of Physiology. Heart and Circulatory Physiology 320 (1): H352–H363. doi:10.1152/ajpheart.00328.2020. PMID 33124885. https://pubmed.ncbi.nlm.nih.gov/33124885/. Retrieved 28 May 2022.
- ↑ Marins, Fernanda Ribeiro; Oliveira, Aline Cristina; Qadri, Fatimunnisa; Motta-Santos, Daisy; Alenina, Natalia; Bader, Michael; Fontes, Marco Antonio Peliky; Santos, Robson Augusto Souza (1 September 2021). "Alamandine but not Ang-(1-7) produces cardiovascular effects at the rostral insular cortex". American Journal of Physiology. Regulatory, Integrative and Comparative Physiology 321 (3): R513–R521. doi:10.1152/ajpregu.00308.2020. PMID 34346721. https://pubmed.ncbi.nlm.nih.gov/34346721/. Retrieved 28 May 2022.
 KSF
KSF