Aluminium carbide
Topic: Chemistry
 From HandWiki - Reading time: 7 min
From HandWiki - Reading time: 7 min
| |
| Names | |
|---|---|
| Preferred IUPAC name
Aluminium carbide | |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
| MeSH | Aluminum+carbide |
PubChem CID
|
|
| UN number | 1394 |
| |
| |
| Properties | |
| Al 4C 3 | |
| Molar mass | 143.95853 g/mol |
| Appearance | colorless (when pure) hexagonal crystals[1] |
| Odor | odorless |
| Density | 2.36 g/cm3[1] |
| Melting point | 2,100 °C (3,810 °F; 2,370 K)[2] |
| Boiling point | decomposes at 1400 °C [3] |
| Evolves methane (CH 4), Al(OH)3, and heat. | |
| Structure | |
| Rhombohedral, hR21, space group[3] | |
| R3m(No. 166) | |
a = 0.3335 nm, b = 0.3335 nm, c = 0.85422 nm α = 78.743°, β = 78.743°, γ = 60°
| |
| Thermochemistry | |
Heat capacity (C)
|
116.8 J/(mol·K) |
Std molar
entropy (S |
88.95 J/(mol·K) |
Std enthalpy of
formation (ΔfH⦵298) |
−209 kJ/mol |
Gibbs free energy (ΔfG˚)
|
−196 kJ/mol |
| Hazards[2] | |
| Safety data sheet | Fisher Science SDS[4] |
| GHS pictograms |  
|
| GHS Signal word | Danger |
| H228, H261, H315, H319, H335 | |
| P223, P231+232, P261, P264, P271, P280, P302+352, P304+340+312Script error: No such module "Preview warning".Category:GHS errors, P305+351+338, P332+313, P335+334, P337+313, P362, P370+378, P402+404, P403+233, P405, P501 | |
| NFPA 704 (fire diamond) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Aluminium carbide is a chemical compound with the chemical formula Al
4C
3. It is a carbide of aluminium. It has the appearance of pale yellow to brown crystals. It is stable up to 1,400 °C (2,550 °F). It decomposes in water with the production of methane.
Preparation
Aluminium carbide is prepared by direct reaction of aluminium and carbon in an electric arc furnace.[5]
- 4 Al + 3 C → Al
4C
3
An alternative reaction begins with alumina, but it is less favorable because of generation of carbon monoxide.
- 2 Al
2O
3 + 9 C → Al
4C
3 + 6 CO
Silicon carbide also reacts with aluminium to yield Al
4C
3. This conversion limits the mechanical applications of SiC, because Al
4C
3 is more brittle than SiC.[6]
- 4 Al + 3 SiC → Al
4C
3 + 3 Si
Reactions
Aluminium carbide hydrolyses with evolution of methane[7] and considerable amounts of heat.[8]
- Al
4C
3 + 12 H
2O → 4 Al(OH)
3 + 3 CH
4
The addition of 10g of Al
4C
3 to 10g of water was found to reach a maximum temperature of 387 °F (197 °C) at a time of 11 minutes in one study, while in another the addition of 20g of Al
4C
3 to 10g of water reached a maximum temperature of 280 °F (138 °C). Reversing the order and adding 10g of water to 20g of Al
4C
3 reached a maximum temperature of 349 °F (176 °C).[8]
Similar reactions occur with other protic reagents:[1]
- Al
4C
3 + 12 HCl → 4 AlCl
3 + 3 CH
4
Composites
Reactive hot isostatic pressing (hipping) at ≈40 megapascals (390 atm) of the appropriate mixtures of Ti, Al
4C
3-graphite, for 15 hours at 1,300 °C (2,370 °F) yields predominantly single-phase samples of Ti
2AlC
0.5N
0.5, 30 hours at 1,300 °C (2,370 °F) yields predominantly single-phase samples of Ti
2AlC (Titanium aluminium carbide).[9]
In aluminium-matrix composites reinforced with silicon carbide, the chemical reactions between silicon carbide and molten aluminium generate a layer of aluminium carbide on the silicon carbide particles, which decreases the strength of the material, although it increases the wettability of the SiC particles.[10] This tendency can be decreased by coating the silicon carbide particles with a suitable oxide or nitride, preoxidation of the particles to form a silica coating, or using a layer of sacrificial metal.[11]
An aluminium-aluminium carbide composite material can be made by mechanical alloying, by mixing aluminium powder with graphite particles.[citation needed]
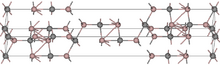
Structure
Aluminium carbide has an unusual crystal structure that consists of alternating layers of Al
2C and Al
2C
2. Each aluminium atom is coordinated to 4 carbon atoms to give a tetrahedral arrangement. Carbon atoms exist in 2 different binding environments; one is a deformed octahedron of 6 Al atoms at a distance of 217 pm. The other is a distorted trigonal bipyramidal structure of 4 Al atoms at 190–194 pm and a fifth Al atom at 221 pm.[5][12]
Occurrence
Small amounts of aluminium carbide are a common impurity of technical calcium carbide. In electrolytic manufacturing of aluminium, aluminium carbide forms as a corrosion product of the graphite electrodes.[13]
In metal matrix composites based on aluminium matrix reinforced with non-metal carbides (silicon carbide, boron carbide, etc.) or carbon fibres, aluminium carbide often forms as an unwanted product. In case of carbon fibre, it reacts with the aluminium matrix at temperatures above 500 °C (932 °F); better wetting of the fibre and inhibition of chemical reaction can be achieved by coating it with e.g. titanium boride.
Applications
Aluminium carbide particles finely dispersed in aluminium matrix lower the tendency of the material to creep, especially in combination with silicon carbide particles.[14]
Aluminium carbide can be used as an abrasive in high-speed cutting tools.[citation needed] It has approximately the same hardness as topaz.[citation needed]
See also
- List of compounds with carbon number 1
References
- ↑ 1.0 1.1 1.2 Mary Eagleson (1994). Concise encyclopedia chemistry. Walter de Gruyter. p. 52. ISBN 978-3-11-011451-5. https://archive.org/details/conciseencyclope00eagl.
- ↑ 2.0 2.1 Sigma-Aldrich Co., Aluminum carbide powder.
- ↑ 3.0 3.1 Gesing, Thorsten M.; Jeitschko, Wolfgang (1 February 1995). "The Crystal Structure and Chemical Properties of U2Al3C4 and Structure Refinement of Al4C3". Zeitschrift für Naturforschung B (Verlag der Zeitschrift für Naturforschung) 50 (2): 196–200. doi:10.1515/znb-1995-0206.
- ↑ 4.0 4.1 "Aluminum carbide SDS" (pdf). Thermo Fisher Scientific. 30 March 2024. p. 3. https://www.fishersci.com/store/msds?partNumber=AA1403830&productDescription=ALUMINUM+CARBIDE+250G&vendorId=VN00024248&countryCode=US&language=en.
- ↑ 5.0 5.1 Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. p. 297. ISBN 978-0-08-037941-8.
- ↑ Deborah D. L. Chung (2010). Composite Materials: Functional Materials for Modern Technologies. Springer. p. 315. ISBN 978-1-84882-830-8. https://books.google.com/books?id=vGstB0vDe04C&pg=PA315.
- ↑ Qualitative Inorganic Analysis. CUP Archive. 1954. p. 102. https://books.google.com/books?id=rzI9AAAAIAAJ&pg=PA102.
- ↑ 8.0 8.1 Kaye, Seymour M.; Herman, Henry L. (1 January 1983). "W - Water, Its Hazardous Reactions and Use in Energetic Materials". Encyclopedia of Explosives and Related Items. Volume 10. Dover, NJ: Picatinny Arsenal. pp. W10-W11. https://apps.dtic.mil/sti/pdfs/ADA134347.pdf?page=309. Retrieved 25 August 2025.
- ↑ Barsoum, M.W.; El-Raghy, T.; Ali, M. (30 June 1999). "Processing and characterization of Ti2AlC, Ti2AlN, and Ti2AlC0.5N0.5". Metallurgical and Materials Transactions A 31 (7): 1857–1865. doi:10.1007/s11661-006-0243-3.
- ↑ Urena; Salazar, Gomez De; Gil; Escalera; Baldonedo (1999). "Scanning and transmission electron microscopy study of the microstructural changes occurring in aluminium matrix composites reinforced with SiC particles during casting and welding: interface reactions". Journal of Microscopy 196 (2): 124–136. doi:10.1046/j.1365-2818.1999.00610.x. PMID 10540265.
- ↑ Guillermo Requena. "A359/SiC/xxp: A359 Al alloy reinforced with irregularly shaped SiC particles". MMC-ASSESS Metal Matrix Composites. http://mmc-assess.tuwien.ac.at/data/prm/duralcan/a359_sic.htm.
- ↑ Solozhenko, Vladimir L.; Kurakevych, Oleksandr O. (2005). "Equation of state of aluminum carbide Al4C3". Solid State Communications 133 (6): 385–388. doi:10.1016/j.ssc.2004.11.030. ISSN 0038-1098. Bibcode: 2005SSCom.133..385S.
- ↑ Thonstad, Jomar; Grjotheim, K.; Malinovský, M.; Malinovský, K.; Krohn, C. (2001). Aluminium electrolysis: fundamentals of the Hall Héroult process (3rd ed.). Düsseldorf, Germany: Aluminium-Verlag Marketing & Kommunikation. p. 314. ISBN 978-3-87017-270-1.
- ↑ Zhu, S.J; Peng, L.M; Zhou, Q; Ma, Z.Y; Kuchařová, K; Čadek, J (August 1999). "Creep behaviour of aluminium strengthened by fine aluminium carbide particles and reinforced by silicon carbide particulates — DS Al–SiC/Al4C3 composites". Materials Science and Engineering: A 268 (1-2): 236–245. doi:10.1016/S0921-5093(98)01119-8.
 |
 KSF
KSF
