Aluminium dihydrogenphosphate
Topic: Chemistry
 From HandWiki - Reading time: 4 min
From HandWiki - Reading time: 4 min
| |
| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| EC Number |
|
PubChem CID
|
|
| |
| |
| Properties | |
| AlH6O12P3 | |
| Molar mass | 317.939 g·mol−1 |
| Appearance | white solid |
| Density | 2.37 g/cm3 |
| Hazards | |
| Safety data sheet | External SDS |
| GHS pictograms |  [1] [1]
|
| H318 | |
| P280, P305+351+338, P310 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Aluminium dihydrogenphosphate describes inorganic compounds with the formula Al(H2PO4)3.xH2O where x = 0 or 3. They are white solids. Upon heating these materials convert sequentially to a family of related polyphosphate salts including aluminium triphosphate (AlH2P3O10.2H2O), aluminium hexametaphosphate (Al2P6O18), and aluminium tetrametaphosphate (Al4(P4O12)3). Some of these materials are used for fireproofing and as ingredients in specialized glasses.[2]
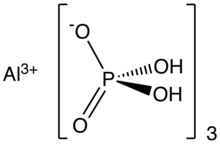
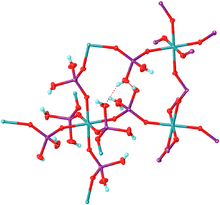
According to analysis by X-ray crystallography, the structure consists of a coordination polymer featuring octahedral Al3+ centers bridged by tetrahedral dihydrogen phosphate ligands. The dihydrogen phosphate ligands are bound to Al3+ as monodentate ligands.[3]
References
- ↑ "Aluminum Phosphate Monobasic". American Elements. https://www.americanelements.com/aluminum-phosphate-monobasic-13530-50-2. Retrieved January 21, 2019.
- ↑ Klaus Schrödter; Gerhard Bettermann; Thomas Staffel; Friedrich Wahl; Thomas Klein; Thomas Hofmann (2008). "Phosphoric Acid and Phosphates". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a19_465.pub3. ISBN 978-3527306732.
- ↑ Brodalla, D.; Kniep, R.; Mootz, D. (1981). "Eine neue Form von Al(H2PO4)3 mit dreidimensionaler Al-O-P Vernetzung". Zeitschrift für Naturforschung B 36: 907–909. doi:10.1515/znb-1981-0803.
 |
Licensed under CC BY-SA 3.0 | Source: https://handwiki.org/wiki/Chemistry:Aluminium_dihydrogenphosphate3 views | ↧ Download this article as ZWI file
 KSF
KSF