Aluminium hydride
Topic: Chemistry
 From HandWiki - Reading time: 14 min
From HandWiki - Reading time: 14 min
| |
| Names | |
|---|---|
| Preferred IUPAC name
Aluminium hydride | |
| Systematic IUPAC name
Alumane | |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| 245 | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| AlH3 | |
| Molar mass | 30.006 g·mol−1 |
| Appearance | white crystalline solid, non-volatile, highly polymerized, needle-like crystals |
| Density | 1.477 g/cm3, solid |
| Melting point | 150 °C (302 °F; 423 K) starts decomposing at 105 °C (221 °F) |
| reacts | |
| Solubility | soluble in ether reacts in ethanol |
| Thermochemistry | |
Heat capacity (C)
|
40.2 J/(mol·K) |
Std molar
entropy (S |
30 J/(mol·K) |
Std enthalpy of
formation (ΔfH⦵298) |
−11.4 kJ/mol |
Gibbs free energy (ΔfG˚)
|
46.4 kJ/mol |
| Related compounds | |
Related compounds
|
Lithium aluminium hydride, diborane |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Aluminium hydride (also known as alane and alumane) is an inorganic compound with the formula AlH
3. Alane and its derivatives are part of a family of common reducing reagents in organic synthesis based around group 13 hydrides.[1] In solution—typically in etherial solvents such tetrahydrofuran or diethyl ether—aluminium hydride forms complexes with Lewis bases, and reacts selectively with particular organic functional groups (e.g., with carboxylic acids and esters over organic halides and nitro groups), and although it is not a reagent of choice, it can react with carbon-carbon multiple bonds (i.e., through hydroalumination). Given its density, and with hydrogen content on the order of 10% by weight,[2] some forms of alane are, as of 2016,[3] active candidates for storing hydrogen and so for power generation in fuel cell applications, including electric vehicles.[not verified in body] As of 2006 it was noted that further research was required to identify an efficient, economical way to reverse the process, regenerating alane from spent aluminium product.
Solid aluminium hydride, or alane, is colorless and nonvolatile, and in its most common reagent form it is a highly polymerized species (i.e., has multiple AlH
3 units that are self-associated); it melts with decomposition at 110 °C.[4] While not spontaneously flammable, alane solids and solutions require precautions in use akin to other highly flammable metal hydrides, and must be handled and stored with the active exclusion of moisture. Alane decomposes on exposure to air (principally because of adventitious moisture), though passivation — here, allowing for development of an inert surface coating — greatly diminishes the rate of decomposition of alane preparations.[not verified in body]
Form and structure
Alane is a colorless and nonvolatile solid that melts with decomposition at 110 °C.[4] The solid form, however, often presents as a white solid that may be tinted grey (with decreasing reagent particle size or increasing impurity levels).[citation needed] This coloration arises from a thin surface passivation layer of aluminium oxide or hydroxide.[citation needed]
Under common laboratory conditions, alane is "highly polymeric", structurally.[4] This is sometimes indicated with the formula (AlH
3)
n, where n is left unspecified.[5]Template:Primary src inline Preparations of alane dissolve in tetrahydrofuran (THF) or diethyl ether (ether),[4] from which pure allotropes precipitate.[6][non-primary source needed]
Structurally, alane can adopt numerous polymorphic forms. By 2006, "at least 7 non-solvated AlH
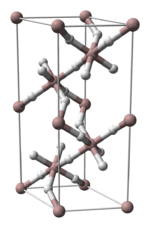
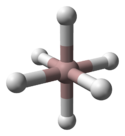
3 phases" were known: α-, α’-, β-, γ-, ε-, and ζ-alanes;[2] the δ- and θ-alanes have subsequently been discovered.[citation needed] Each has a different structure, with α-alane being the most thermally stable polymorph.[citation needed] For instance, crystallographically, α-alane adopts a cubic or rhombohedral morphology, while α’-alane forms needle-like crystals and γ-alane forms bundles of fused needles.[citation needed] The crystal structure of α-alane has been determined, and features aluminium atoms surrounded by six octahedrally oriented hydrogen atoms that bridge to six other aluminium atoms (see table), where the Al-H distances are all equivalent (172 pm) and the Al-H-Al angle is 141°.[7]
| Crystallographic Structure of α-AlH 3[8] | |||||
|---|---|---|---|---|---|
| The α-AlH 3 unit cell |
Aluminium coordination | Hydrogen coordination | |||
|
|

| |||
When β- and γ-alanes are produced together, they convert to α-alane upon heating, while δ-, ε-, and θ-alanes are produced in still other crystallization conditions; although they are less thermally stable, the δ-, ε-, and θ-alane polymorphs do not convert to α-alane upon heating.[6][better source needed]
Under special conditions, non-polymeric alanes (i.e., molecular forms of it) can be prepared and studied. Monomeric AlH
3 has been isolated at low temperature in a solid noble gas matrix where it was shown to be planar.[9] The dimeric form, Al
2H
6, has been isolated in solid hydrogen, and it is isostructural with diborane (B
2H
6) and digallane (Ga
2H
6).[10][11][non-primary source needed]
Handling
Alane is not spontaneously flammable.[12] Even so, "similar handling and precautions as... exercised for Li[AlH
4]" (the chemical reagent, lithium aluminium hydride) are recommended, as its "reactivity [is] comparable" to this related reducing reagent.[4] For these reagents, both preparations in solutions and isolated solids are "highly flammable and must be stored in the absence of moisture".[13] Laboratory guides recommend alane use inside a fume hood.[4][why?] Solids of this reagent type carry recommendations of handling "in a glove bag or dry box".[13] After use, solution containers are typically sealed tightly with concomitant flushing with inert gas to exclude the oxygen and moisture of ambient air.[13]
Passivation[clarification needed] greatly diminishes the decomposition rate associated with alane preparations.[citation needed] Passivated alane nevertheless retains a hazard classification of 4.3 (chemicals which in contact with water, emit flammable gases).[14]
Preparation
Aluminium hydrides and various complexes thereof have long been known.[15] Its first synthesis was published in 1947, and a patent for the synthesis was assigned in 1999.[16][17] Aluminium hydride is prepared by treating lithium aluminium hydride with aluminium trichloride.[18] The procedure is intricate: attention must be given to the removal of lithium chloride.
- 3 Li[AlH
4] + AlCl
3 → 4 AlH
3 + 3 LiCl
The ether solution of alane requires immediate use, because polymeric material rapidly precipitates as a solid. Aluminium hydride solutions are known to degrade after 3 days. Aluminium hydride is more reactive than Li[AlH
4].[6]
Several other methods exist for the preparation of aluminium hydride:
- 2 Li[AlH
4] + BeCl
2 → 2 AlH
3 + Li
2[BeH
2Cl
2] - 2 Li[AlH
4] + H
2SO
4 → 2 AlH
3 + Li
2SO
4 + 2 H
2 - 2 Li[AlH
4] + ZnCl
2 → 2 AlH
3 + 2 LiCl + ZnH
2 - 2 Li[AlH
4] + I
2 → 2 AlH
3 + 2 LiI + H
2
Electrochemical synthesis
Several groups have shown that alane can be produced electrochemically.[19][20][21][22][23] Different electrochemical alane production methods have been patented.[24][25] Electrochemically generating alane avoids chloride impurities. Two possible mechanisms are discussed for the formation of alane in Clasen's electrochemical cell containing THF as the solvent, sodium aluminium hydride as the electrolyte, an aluminium anode, and an iron (Fe) wire submerged in mercury (Hg) as the cathode. The sodium forms an amalgam with the Hg cathode preventing side reactions and the hydrogen produced in the first reaction could be captured and reacted back with the sodium mercury amalgam to produce sodium hydride. Clasen's system results in no loss of starting material. For insoluble anodes, reaction 1 occurs, while for soluble anodes, anodic dissolution is expected according to reaction 2:
- [AlH
4]−
− e−
+ n THF → AlH
3 · nTHF + 1/
2 H
2 - 3 [AlH
4]−
+ Al − 3 e−
+ 4n THF → 4 AlH
3 · nTHF
In reaction 2, the aluminium anode is consumed, limiting the production of aluminium hydride for a given electrochemical cell.
The crystallization and recovery of aluminium hydride from electrochemically generated alane has been demonstrated.[22][23]
High pressure hydrogenation of aluminium metal
α-AlH
3 can be produced by hydrogenation of aluminium metal at 10 GPa and 600 °C (1,112 °F). The reaction between the liquified hydrogen produces α-AlH
3 which could be recovered under ambient conditions.[26]
Reactions
Formation of adducts with Lewis bases
AlH
3 readily forms adducts with strong Lewis bases. For example, both 1:1 and 1:2 complexes form with trimethylamine. The 1:1 complex is tetrahedral in the gas phase,[27] but in the solid phase it is dimeric with bridging hydrogen centres, (N(CH
3)
3Al(μ-H))
2.[28] The 1:2 complex adopts a trigonal bipyramidal structure.[27] Some adducts (e.g. dimethylethylamine alane, (CH
3CH
2)(CH
3)
2N · AlH
3) thermally decompose to give aluminium metal and may have use in MOCVD applications.[29]
Its complex with diethyl ether forms according to the following stoichiometry:
- AlH
3 + (CH
3CH
2)
2O → (CH
3CH
2)
2O · AlH
3
The reaction with lithium hydride in ether produces lithium aluminium hydride:
- AlH
3 + LiH → Li[AlH
4]
Reduction of functional groups
In organic chemistry, aluminium hydride is mainly used for the reduction of functional groups.[30] In many ways, the reactivity of aluminium hydride is similar to that of lithium aluminium hydride. Aluminium hydride will reduce aldehydes, ketones, carboxylic acids, anhydrides, acid chlorides, esters, and lactones to their corresponding alcohols. Amides, nitriles, and oximes are reduced to their corresponding amines.
In terms of functional group selectivity, alane differs from other hydride reagents. For example, in the following cyclohexanone reduction, lithium aluminium hydride gives a trans:cis ratio of 1.9 : 1, whereas aluminium hydride gives a trans:cis ratio of 7.3 : 1.[31]
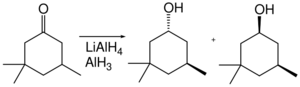
Alane enables the hydroxymethylation of certain ketones (that is the replacement of C–H by C–CH
2OH at the alpha position).[32] The ketone itself is not reduced as it is "protected" as its enolate.
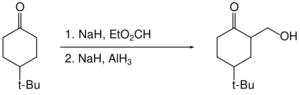
Organohalides are reduced slowly or not at all by aluminium hydride. Therefore, reactive functional groups such as carboxylic acids can be reduced in the presence of halides.[33]
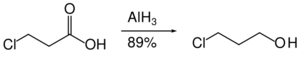
Nitro groups are not reduced by aluminium hydride. Likewise, aluminium hydride can accomplish the reduction of an ester in the presence of nitro groups.[34]
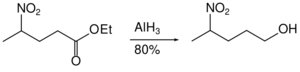
Aluminium hydride can be used in the reduction of acetals to half protected diols.[35]
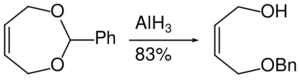
Aluminium hydride can also be used in epoxide ring opening reaction as shown below.[36]

The allylic rearrangement reaction carried out using aluminium hydride is a SN2 reaction, and it is not sterically demanding.[37]
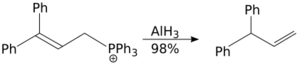
Aluminium hydride will reduce carbon dioxide to methane with heating:[citation needed]
- 4 AlH
3 + 3 CO
2 → 3 CH
4 + 2 Al
2O
3
Aluminium hydride has been shown to add to propargylic alcohols.[38] Akin to hydroboration, aluminium hydride can, in the presence of titanium tetrachloride, add across double bonds.[39]

Fuel
In its passivated form, alane is an active candidate for storing hydrogen, and can be used for efficient power generation via fuel cell applications, including fuel cell and electric vehicles and other lightweight power applications.[citation needed] AlH
3 contains up 10.1% hydrogen by weight (at a density of 1.48 grams per milliliter),[2]. As of 2006, AlH
3 was being described as a candidate for which "further research w[ould] be required to develop an efficient and economical process to regenerate [it] from the spent Al powder".[2][needs update]
Allane is also a potential additive to rocket fuel and in explosive and pyrotechnic compositions.[citation needed] In its unpassivated form, alane is also a promising rocket fuel additive, capable of delivering impulse efficiency gains of up to 10%.[40]
Reported accidents
A reduction of trifluoromethyl compound with alane was reported to have caused a "[s]erious [e]xplosion".[41]
References
- ↑ Brown, H. C.; Krishnamurthy, S. (1979). "Forty Years of Hydride Reductions". Tetrahedron 35 (5): 567–607. doi:10.1016/0040-4020(79)87003-9.
- ↑ 2.0 2.1 2.2 2.3 Graetz, J..; Reilly, J..; Sandrock, G..; Johnson, J..; Zhou, W.-M.; Wegrzyn, J. (2006). Aluminum Hydride, A1H3, As a Hydrogen Storage Compound (Report). Washington, D.C.: Office of Science and Technical Information [OSTI]. doi:10.2172/899889. https://www.osti.gov/biblio/899889-UGd8IT/. Retrieved 28 July 2022.
- ↑ Lin-Lin Wang; Aditi Herwadkar; Jason M. Reich; Duane D. Johnson; Stephen D. House; Pamela Peña-Martin; Angus A. Rockett; Ian M. Robertson et al. (2016). "Towards Direct Synthesis of Alane: A Predicted Defect-Mediated Pathway Confirmed Experimentally". ChemSusChem 9 (17): 2358–2364. doi:10.1002/cssc.201600338. PMID 27535100. Bibcode: 2016ChSCh...9.2358W. https://chemistry-europe.onlinelibrary.wiley.com/doi/10.1002/cssc.201600338.
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 Galatsis, P; Sintim, Herman O.; Wang J. (15 September 2008). "Aluminum Hydride". Encyclopedia of Reagents for Organic Synthesis (online ed.). New York, N.Y.: John Wiley & Sons. doi:10.1002/047084289X.ra082.pub2. ISBN 978-0471936237. https://onlinelibrary.wiley.com/doi/10.1002/047084289X.ra082.pub2. Retrieved 28 July 2022.
- ↑ See, e.g., Andrews & Wang 2003.
- ↑ 6.0 6.1 6.2 Lund, G. K.; Hanks, J. M.; Johnston, H. E., "Method for the Production of α-Alane.", US patent application 2007066839
- ↑ Turley & Rinn 1969. (Abstract) "The final Al⋯H distance of 1.72 Å, the participation of each Al in six bridges, and the equivalence of all Al⋯H distances suggest that 3c-2e bonding occurs." Angle is lasted as "Al(6)-H(5)-Al(4)" in Table IV.
- ↑ Turley, J. W.; Rinn, H. W. (1969). "The Crystal Structure of Aluminum Hydride". Inorganic Chemistry 8 (1): 18–22. doi:10.1021/ic50071a005.
- ↑ Kurth, F. A.; Eberlein, R. A.; Schnöckel, H.-G.; Downs, A. J.; Pulham, C. R. (1993). "Molecular Aluminium Trihydride, AlH3: Generation in a Solid Noble Gas Matrix and Characterisation by its Infrared Spectrum and ab initio Calculations". Journal of the Chemical Society, Chemical Communications 1993 (16): 1302–1304. doi:10.1039/C39930001302. (Abstract) Broad-band photolysis of a solid noble gas matrix containing Al atoms and H2 gives rise to the planar, monomeric AlH3 molecule.
- ↑ Andrews, Lester; Wang Xuefeng (2003). "The Infrared Spectrum of Al2H6 in Solid Hydrogen". Science 299 (5615): 2049–2052. doi:10.1126/science.1082456. PMID 12663923. Bibcode: 2003Sci...299.2049A. See also emendations at doi:10.1126/science.300.5620.741a.
- ↑ Pulham, C. R.; Downs, A. J.; Goode, M. J.; Rankin D. W. H.; Robertson, H. E. (1991). "Gallane: Synthesis, Physical and Chemical Properties, and Structure of the Gaseous Molecule Ga2H6 as Determined by Electron Diffraction". Journal of the American Chemical Society 113 (14): 5149–5162. doi:10.1021/ja00014a003.
- ↑ Galatsis, Sintim & Wang 2008, which describes the phenomenon using the synonym "inflammable".
- ↑ 13.0 13.1 13.2 Paquette, L. A.; Ollevier, T.; Desyroy, V. (15 October 2004). "Lithium Aluminum Hydride". Encyclopedia of Reagents for Organic Synthesis (online ed.). New York, N.Y.: John Wiley & Sons. doi:10.1002/047084289X.rl036.pub2. ISBN 0471936235. https://onlinelibrary.wiley.com/doi/10.1002/047084289X.rl036.pub2. Retrieved 28 July 2022.
- ↑ 2013 CFR Title 29 Volume 6 Section 1900.1200 Appendix B.12
- ↑ Brower, F. M.; Matzek, N. E.; Reigler, P. F.; Rinn, H. W.; Schmidt, D. L.; Snover, J. A.; Terada, K. (1976). "Preparation and Properties of Aluminum Hydride". Journal of the American Chemical Society 98 (9): 2450–2454. doi:10.1021/ja00425a011.
- ↑ Finholt, A. E.; Bond, A. C. Jr.; Schlesinger, H. I. (1947). "Lithium Aluminum Hydride, Aluminum Hydride and Lithium Gallium Hydride, and Some of their Applications in Organic and Inorganic Chemistry". Journal of the American Chemical Society 69 (5): 1199–1203. doi:10.1021/ja01197a061.
- ↑ Petrie, M. A.; Bottaro, J. C.; Schmitt, R. J.; Penwell, P. E.; Bomberger, D. C., "Preparation of Aluminum Hydride Polymorphs, Particularly Stabilized α-AlH3", US patent 6228338, issued 2001-05-08
- ↑ Schmidt, D. L.; Roberts, C. B.; Reigler, P. F.; Lemanski, M. F. Jr.; Schram, E. P. (1973). "Aluminum Trihydride-diethyl etherate ( Etherated Alane )". Inorganic Syntheses. 14. pp. 47–52. doi:10.1002/9780470132456.ch10. ISBN 9780470132456.
- ↑ Alpatova, N. M.; Dymova, T. N.; Kessler, Yu. M.; Osipov, O. R. (1968). "Physicochemical Properties and Structure of Complex Compounds of Aluminium Hydride". Russian Chemical Reviews 37 (2): 99–114. doi:10.1070/RC1968v037n02ABEH001617. Bibcode: 1968RuCRv..37...99A.
- ↑ Semenenko, K. N.; Bulychev, B. M.; Shevlyagina, E. A. (1966). "Aluminium Hydride". Russian Chemical Reviews 35 (9): 649–658. doi:10.1070/RC1966v035n09ABEH001513. Bibcode: 1966RuCRv..35..649S.
- ↑ Osipov, O. R.; Alpatova, N. M.; Kessler, Yu. M. (1966). "none". Elektrokhimiya 2: 984.
- ↑ 22.0 22.1 Zidan, R.; Garcia-Diaz, B. L.; Fewox, C. S.; Stowe, A. C.; Gray, J. R.; Harter, A. G. (2009). "Aluminium hydride: a reversible material for hydrogen storage". Chemical Communications (25): 3717–3719. doi:10.1039/B901878F. PMID 19557259. https://zenodo.org/record/1229996.
- ↑ 23.0 23.1 Martinez-Rodriguez, M. J.; Garcia-Diaz, B. L.; Teprovich, J. A.; Knight, D. A.; Zidan, R. (2012). "Advances in the electrochemical regeneration of aluminum hydride". Applied Physics A: Materials Science & Processing 106 (25): 545–550. doi:10.1007/s00339-011-6647-y. Bibcode: 2012ApPhA.106..545M.
- ↑ Clasen, H., "Verfahren zur Herstellung von Aluminiumhydrid bzw. aluminiumwasserstoffreicher komplexer Hydride", DE patent 1141623, issued 1962-12-27, assigned to Metallgesellschaft
- ↑ Zidan, R., "Electrochemical process and production of novel complex hydrides", US patent 8470156, issued 2013-06-25, assigned to Savannah River Nuclear Solutions, LLC
- ↑ Saitoh, H; Sakurai, Y; Machida, A; Katayama, Y; Aoki, K (2010). "In situX-ray diffraction measurement of the hydrogenation and dehydrogenation of aluminum and characterization of the recovered AlH3". Journal of Physics: Conference Series 215 (1): 012127. doi:10.1088/1742-6596/215/1/012127. ISSN 1742-6596. Bibcode: 2010JPhCS.215a2127S.
- ↑ 27.0 27.1 Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- ↑ Atwood, J. L.; Bennett, F. R.; Elms, F. M.; Jones, C.; Raston, C. L.; Robinson, K. D. (1991). "Tertiary Amine Stabilized Dialane". Journal of the American Chemical Society 113 (21): 8183–8185. doi:10.1021/ja00021a063.
- ↑ Yun, J.-H.; Kim, B.-Y.; Rhee, S.-W. (1998). "Metal-Organic Chemical Vapor Deposition of Aluminum from Dimethylethylamine Alane". Thin Solid Films 312 (1–2): 259–263. doi:10.1016/S0040-6090(97)00333-7. Bibcode: 1998TSF...312..259Y.
- ↑ Galatsis, P. (2001). Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/047084289X.rd245. ISBN 978-0-470-84289-8.
- ↑ Ayres, D. C.; Sawdaye, R. (1967). "The Stereoselective Reduction of Ketones by Aluminium Hydride". Journal of the Chemical Society B 1967: 581–583. doi:10.1039/J29670000581.
- ↑ Corey, E. J.; Cane, D. E. (1971). "Controlled Hydroxymethylation of Ketones". Journal of Organic Chemistry 36 (20): 3070. doi:10.1021/jo00819a047.
- ↑ Jorgenson, Margaret J. (July 1962). "Selective reductions with aluminum hydride". Tetrahedron Letters 3 (13): 559–562. doi:10.1016/S0040-4039(00)76929-2.
- ↑ Takano, S.; Akiyama, M.; Sato, S.; Ogasawara, K. (1983). "A Facile Cleavage of Benzylidene Acetals with Diisobutylaluminum Hydride" (pdf). Chemistry Letters 12 (10): 1593–1596. doi:10.1246/cl.1983.1593. http://www.jstage.jst.go.jp/article/cl1972/12/10/12_10_1593/_pdf.
- ↑ Richter, W. J. (1981). "Asymmetric Synthesis at Prochiral Centers: Substituted 1,3-Dioxolanes". Journal of Organic Chemistry 46 (25): 5119–5124. doi:10.1021/jo00338a011.
- ↑ Maruoka, K.; Saito, S.; Ooi, T.; Yamamoto, H. (1991). "Selective Reduction of Methylenecycloalkane Oxides with 4-Substituted Diisobutylaluminum 2,6-Di-tert-butylphenoxides". Synlett 1991 (4): 255–256. doi:10.1055/s-1991-20698.
- ↑ Claesson, A.; Olsson, L.-I. (1979). "Allenes and Acetylenes. 22. Mechanistic Aspects of the Allene-Forming Reductions (SN2' Reaction) of Chiral Propargylic Derivatives with Hydride Reagents". Journal of the American Chemical Society 101 (24): 7302–7311. doi:10.1021/ja00518a028.
- ↑ Corey, E. J.; Katzenellenbogen, J. A.; Posner, G. H. (1967). "New Stereospecific Synthesis of Trisubstituted Olefins. Stereospecific Synthesis of Farnesol". Journal of the American Chemical Society 89 (16): 4245–4247. doi:10.1021/ja00992a065.
- ↑ Sato, F.; Sato, S.; Kodama, H.; Sato, M. (1977). "Reactions of Lithium Aluminum Hydride or Alane with Olefins Catalyzed by Titanium Tetrachloride or Zirconium Tetrachloride. A Convenient Route to Alkanes, 1-Haloalkanes and Terminal Alcohols from Alkenes". Journal of Organometallic Chemistry 142 (1): 71–79. doi:10.1016/S0022-328X(00)91817-5.
- ↑ Calabro, M. (2011). "Overview of Hybrid Propulsion". Progress in Propulsion Physics 2: 353–374. doi:10.1051/eucass/201102353. ISBN 978-2-7598-0673-7. Bibcode: 2011EUCAS...2..353C.
- ↑ Taydakov, Ilya V. (2020-07-08). "Serious Explosion during Large-Scale Preparation of an Amine by Alane (AlH3) Reduction of a Nitrile Bearing a CF3 Group". ACS Chemical Health & Safety (American Chemical Society (ACS)) 27 (4): 235–239. doi:10.1021/acs.chas.0c00045. ISSN 1871-5532.
External links
- Aluminium Hydride on EnvironmentalChemistry.com Chemical Database
- Hydrogen Storage from Brookhaven National Laboratory
- Aluminum Trihydride on WebElements
 |
 KSF
KSF






