Anthrone
Topic: Chemistry
 From HandWiki - Reading time: 2 min
From HandWiki - Reading time: 2 min
Anthrone is a tricyclic aromatic ketone. It is used for a common cellulose assay and in the colorimetric determination of carbohydrates.[1]
Derivatives of anthrone are used in pharmacy as laxative. They stimulate the motion of the colon and reduce water reabsorption. Some anthrone derivatives can be extracted from a variety of plants, including Rhamnus frangula, Aloe ferox, Rheum officinale, and Cassia senna.[2] Glycosides of anthrone are also found in high amounts in rhubarb leaves, and alongside concentrated amounts of oxalic acid are the reason for the leaves being inedible.
Synthesis and reactions
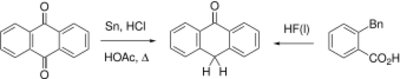
Anthrone can be prepared from anthraquinone by reduction with tin or copper.[3]
An alternative synthesis involves cyclization of o-benzylbenzoic acid induced with hydrogen fluoride.[4]
Anthrone condenses with glyoxal to give, following dehydrogenation, acedianthrone, a useful octacyclic pigment.[5]

Anthrone is the more stable tautomer relative to the anthrol as has been established also by X-ray crystallography.[6] The tautomeric equilibrium is estimated at 100 in aqueous solution. For the two other isomeric anthrols, the tautomeric equilibrium is reversed: they are phenolic.[7]
Anthrone undergoes nitration using conventional conditions for aromatic nitration, implying that it is the hydroxy tautomer that is the reactant.[8]
References
- ↑ Trevelyan, W. E.; Forrest, RS; Harrison, JS (1952). "Determination of Yeast Carbohydrates with the Anthrone Reagent". Nature 170 (4328): 626–627. doi:10.1038/170626a0. PMID 13002392. Bibcode: 1952Natur.170..626T.
- ↑ Niaz, Kamal; Khan, Fazlullah (2020-01-01), Sanches Silva, Ana; Nabavi, Seyed Fazel; Saeedi, Mina et al., eds., "Chapter 3 - Analysis of polyphenolics", Recent Advances in Natural Products Analysis (Elsevier): pp. 39–197, doi:10.1016/b978-0-12-816455-6.00003-2, ISBN 978-0-12-816455-6, https://www.sciencedirect.com/science/article/pii/B9780128164556000032, retrieved 2024-06-01
- ↑ Macleod, L. C.; Allen, C. F. H. (1934). "Benzanthrone". Organic Syntheses 14: 4. doi:10.15227/orgsyn.014.0004.
- ↑ Fieser, Louis F.; Hershberg, E. B. (May 1939). "Inter- and Intramolecular Acylations with Hydrogen Fluoride". Journal of the American Chemical Society 61 (5): 1272–1281. doi:10.1021/ja01874a079. Bibcode: 1939JAChS..61.1272F.
- ↑ Bien, H.-S.; Stawitz, J.; Wunderlich, K. (2005). "Ullmann's Encyclopedia of Industrial Chemistry". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a02_355.
- ↑ Lian, Jian-Jou; Lin, Chung-Chang; Chang, Hsu-Kai; Chen, Po-Chiang; Liu, Rai-Shung (2006). "Thermal and Metal-Catalyzed Cyclization of 1-Substituted 3,5-Dien-1-ynes via a [1,7]-Hydrogen Shift: Development of a Tandem Aldol Condensation−Dehydration and Aromatization Catalysis between 3-En-1-yn-5-al Units and Cyclic Ketones". Journal of the American Chemical Society 128 (30): 9661–9667. doi:10.1021/ja061203b. PMID 16866518. Bibcode: 2006JAChS.128.9661L.
- ↑ Ośmiałowski, Borys; Raczyńska, Ewa D.; Krygowski, Tadeusz M. (2006). "Tautomeric Equilibria and Pi Electron Delocalization for Some Monohydroxyarenes Quantum Chemical Studies". The Journal of Organic Chemistry 71 (10): 3727–3736. doi:10.1021/jo052615q. PMID 16674042.
- ↑ Kurt H. Meyer (1928). "Nitroanthrone". Organic Syntheses 8: 78. doi:10.15227/orgsyn.008.0078.
 |
 KSF
KSF