Arsenate
Topic: Chemistry
 From HandWiki - Reading time: 5 min
From HandWiki - Reading time: 5 min
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Arsenate
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
PubChem CID
|
|||
| UNII | |||
| |||
| |||
| Properties | |||
| AsO3− 4 | |||
| Molar mass | 138.918 g·mol−1 | ||
| Conjugate acid | Arsenic acid | ||
| Hazards | |||
| Main hazards | Extremely toxic, carcinogenic | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
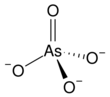
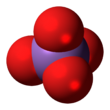
The arsenate is an ion with the chemical formula AsO3−
4.[1] Bonding in arsenate consists of a central arsenic atom, with oxidation state +5, double bonded to one oxygen atom and single bonded to a further three oxygen atoms.[2] The four oxygen atoms orient around the arsenic atom in a tetrahedral geometry.[2] Resonance disperses the ion's −3 charge across all four oxygen atoms.
Arsenate readily reacts with metals to form arsenate metal compounds.[2][3] Arsenate is a moderate oxidizer and an electron acceptor, with an electrode potential of +0.56 V for its reduction to arsenite.[4] Due to arsenic having the same valency and similar atomic radius to phosphorus, arsenate shares similar geometry and reactivity with phosphate.[5] Arsenate can replace phosphate in biochemical reactions and is toxic to most organisms.[5][6]
Natural occurrence

Arsenates occur naturally, in hydrated and anhydrous form, in a variety of minerals. Examples of arsenate-containing minerals include adamite, alarsite, annabergite, erythrite and legrandite.[7] When two arsenate ions balance the charge in a formula, it is called diarsenate for example zinc diarsenate, Zn
3(AsO
4)
2.
Uses
Arsenate-based pesticides such as lead hydrogen arsenate were commonly used until their replacement by newer pesticides such as DDT and subsequent ban by multiple regulatory bodies due to health concerns.[8][9]
Transition metal arsenate compounds are often brightly coloured and have been used to make pigments. Copper arsenate was a minor compound used in the Egyptian blue pigment used by the ancient Egyptians and Romans.[10] Cobalt violet pigment was made from cobalt arsenate before its toxicity led to its replacement by cobalt phosphate.[11][12][13]
Chromated copper arsenate (CCA) has been a widely used wood preservative since the 1930s.[14] Safety concerns have led to the phasing out of CCA-treated wood for residential projects in many countries.[14] CCA remains a common and economical treatment choice for non-residential uses such as agriculture. [14][15]
Speciation

Depending on the pH, arsenate can be found as trihydrogen arsenate (that is arsenic acid H
3AsO
4), dihydrogen arsenate (H
2AsO−
4), hydrogen arsenate (HAsO2−
4), or arsenate (AsO3−
4).[18] Trihydrogen arsenate is also known as arsenic acid. At a given pH, the distribution of these arsenate species can be determined from their respective acid dissociation constants.[17]
- H
3AsO
4 + H
2O ⇌ H
2AsO−
4 + [H
3O]+
(pKa1 = 2.19) - H
2AsO−
4 + H
2O ⇌ HAsO2−
4 + [H
3O]+
(pKa2 = 6.94) - HAsO2−
4 + H
2O ⇌ AsO3−
4 + [H
3O]+
(pKa3 = 11.5)
These values are similar to those of phosphoric acid. Hydrogen arsenate and dihydrogen arsenate predominate in aqueous solution near neutral pH.[17]
The reduction potential (pe) of a solution also affects arsenate speciation. In natural waters, the dissolved oxygen content is the main factor influencing reduction potential. Arsenates occur in oxygenated waters, which have a high pe, while arsenites are the main arsenic species in anoxic waters with a low pe.[16]
A Pourbaix diagram shows the combined influence of pH and pe on arsenate speciation.
Contamination
Arsenates, along with arsenites, are a significant source of contamination in some natural water sources and can lead to arsenic poisoning with repeated exposure.[19][20] Countries with high levels of arsenic minerals in sediment and rock, such as Bangladesh, are especially at risk of arsenate contamination.[21][20]
Arsenate poisoning
Arsenate is harmful to humans and animals as it interferes with the normal functioning of glycolysis and the Krebs cycle. Arsenate replaces inorganic phosphate in the step of glycolysis that produces 1,3-bisphosphoglycerate from glyceraldehyde 3-phosphate. This yields 1-arseno-3-phosphoglycerate instead, which is unstable and quickly hydrolyzes, forming the next intermediate in the pathway, 3-phosphoglycerate. Therefore, glycolysis proceeds, but the ATP molecule that would be generated from 1,3-bisphosphoglycerate is lost – arsenate is an uncoupler of glycolysis, explaining its toxicity.[22][23]
As with other arsenic compounds, arsenate binds to lipoic acid, inhibiting the conversion of pyruvate into acetyl-CoA, blocking the Krebs cycle and therefore resulting in further loss of ATP.[23]
See also
References
- ↑ PubChem. "Arsenate ion" (in en). https://pubchem.ncbi.nlm.nih.gov/compound/27401.
- ↑ 2.0 2.1 2.2 "Arsenate mineral | Britannica" (in en). https://www.britannica.com/science/arsenate-mineral.
- ↑ Waalkes, Michael P. (2019), Baan, Robert A.; Stewart, Bernard W.; Straif, Kurt, eds., "Arsenic and metals", Tumour Site Concordance and Mechanisms of Carcinogenesis, IARC Scientific Publications (Lyon (FR): International Agency for Research on Cancer), ISBN 978-92-832-2217-0, PMID 33979075, http://www.ncbi.nlm.nih.gov/books/NBK570318/, retrieved 2023-04-02
- ↑ "P1: Standard Reduction Potentials by Element" (in en). 2013-12-02. https://chem.libretexts.org/Ancillary_Materials/Reference/Reference_Tables/Electrochemistry_Tables/P1%3A_Standard_Reduction_Potentials_by_Element.
- ↑ 5.0 5.1 Pollutants, National Research Council (US) Committee on Medical and Biological Effects of Environmental (1977) (in en). Chemistry of Arsenic. National Academies Press (US). https://www.ncbi.nlm.nih.gov/books/NBK231019/.
- ↑ Elias, Mikael; Wellner, Alon; Goldin-Azulay, Korina; Chabriere, Eric; Vorholt, Julia A.; Erb, Tobias J.; Tawfik, Dan S. (2012). "The molecular basis of phosphate discrimination in arsenate-rich environments" (in en). Nature 491 (7422): 134–137. doi:10.1038/nature11517. ISSN 1476-4687. PMID 23034649. Bibcode: 2012Natur.491..134E. https://www.nature.com/articles/nature11517.
- ↑ "The mineralogy of Arsenic". https://www.mindat.org/element/Arsenic.
- ↑ "The Evolution of Chemical Pesticides". https://www.fishersci.ca/ca/en/publications/lab-reporter/2016/issue-4/the-evolution-chemical-pesticides.html.
- ↑ "The Global Problem of Lead Arsenate Pesticide". https://lead.org.au/lanv10n3/lanv10n3-7.html.
- ↑ Dariz, Petra; Schmid, Thomas (2021-05-28). "Trace compounds in Early Medieval Egyptian blue carry information on provenance, manufacture, application, and ageing" (in en). Scientific Reports 11 (1): 11296. doi:10.1038/s41598-021-90759-6. ISSN 2045-2322. PMID 34050218. Bibcode: 2021NatSR..1111296D.
- ↑ Corbeil, Marie-Claude; Charland, Jean-Pierre; Moffatt, Elizabeth A. (2002). "The Characterization of Cobalt Violet Pigments". Studies in Conservation 47 (4): 237–249. doi:10.2307/1506784. ISSN 0039-3630. https://www.jstor.org/stable/1506784.
- ↑ "Cobalt violet" (in en-US). https://colourlex.com/project/cobalt-violet/.
- ↑ "Cobaltous arsenate - CAMEO" (in en). https://cameo.mfa.org/wiki/Cobaltous_arsenate.
- ↑ 14.0 14.1 14.2 Barton, C. (2014-01-01), Wexler, Philip, ed. (in en), CCA-Treated Wood, Oxford: Academic Press, pp. 751–752, ISBN 978-0-12-386455-0, https://www.sciencedirect.com/science/article/pii/B9780123864543002724, retrieved 2023-04-10
- ↑ Likar, M; Schauer, P; Japelj, M; Globokar, M; Oklobdzija, M; Povse, A; Sunjić, V (1970-01-01). "Synthesis and antimicrobial activity of some thenoyl amides". Journal of Medicinal Chemistry 13 (1): 159–161. doi:10.1021/jm00295a053. ISSN 1520-4804. PMID 5412102. https://doi.org/10.1021/jm00295a053.
- ↑ 16.0 16.1 Marinho, Belisa A.; Cristóvão, Raquel O.; Boaventura, Rui A. R.; Vilar, Vítor J. P. (2019-01-01). "As(III) and Cr(VI) oxyanion removal from water by advanced oxidation/reduction processes—a review" (in en). Environmental Science and Pollution Research 26 (3): 2203–2227. doi:10.1007/s11356-018-3595-5. ISSN 1614-7499. PMID 30474808. https://doi.org/10.1007/s11356-018-3595-5.
- ↑ 17.0 17.1 17.2 Jekel, M.; Amy, G. L. (2006-01-01), Newcombe, Gayle; Dixon, David, eds., "Chapter 11 - Arsenic removal during drinking water treatment" (in en), Interface Science and Technology, Interface Science in Drinking Water Treatment (Elsevier) 10: pp. 193–206, https://www.sciencedirect.com/science/article/pii/S1573428506800803, retrieved 2023-04-15
- ↑ Pollutants, National Research Council (US) Committee on Medical and Biological Effects of Environmental (1977) (in en). Chemistry of Arsenic. National Academies Press (US). https://www.ncbi.nlm.nih.gov/books/NBK231019/.
- ↑ Sánchez-Rodas, Daniel; Luis Gómez-Ariza, José; Giráldez, Inmaculada; Velasco, Alfredo; Morales, Emilio (2005-06-01). "Arsenic speciation in river and estuarine waters from southwest Spain". The Science of the Total Environment 345 (1–3): 207–217. doi:10.1016/j.scitotenv.2004.10.029. ISSN 0048-9697. PMID 15919540. Bibcode: 2005ScTEn.345..207S. https://pubmed.ncbi.nlm.nih.gov/15919540/.
- ↑ 20.0 20.1 "Arsenic" (in en). https://www.who.int/news-room/fact-sheets/detail/arsenic.
- ↑ UCL (2022-05-10). "Reducing population exposure to groundwater arsenic in Bangladesh" (in en). https://www.ucl.ac.uk/earth-sciences/news/2022/may/reducing-population-exposure-groundwater-arsenic-bangladesh.
- ↑ "How does arsenic kill?" (in en). https://www.livescience.com/how-does-arsenic-kill.
- ↑ 23.0 23.1 Hughes, Michael F. (2002-07-07). "Arsenic toxicity and potential mechanisms of action". Toxicology Letters 133 (1): 1–16. doi:10.1016/s0378-4274(02)00084-x. ISSN 0378-4274. PMID 12076506. https://pubmed.ncbi.nlm.nih.gov/12076506/.
 |
 KSF
KSF