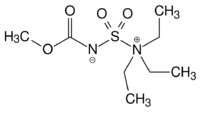
Burgess reagent
Topic: Chemistry
 From HandWiki - Reading time: 2 min
From HandWiki - Reading time: 2 min
| |
| Names | |
|---|---|
| IUPAC name
1-Methoxy-N-triethylammoniosulfonyl-methanimidate
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C8H18N2O4S | |
| Molar mass | 238.30 g·mol−1 |
| Hazards | |
| GHS pictograms | 
|
| GHS Signal word | Warning |
| H315, H319, H335 | |
| P261, P264, P271, P280, P302+352, P304+340, P305+351+338, P312, P321, P332+313, P337+313, P362, P403+233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
The Burgess reagent (methyl N-(triethylammoniumsulfonyl)carbamate) is a mild and selective dehydrating reagent often used in organic chemistry.[1][2] It was developed in the laboratory of Edward M. Burgess at Georgia Tech.
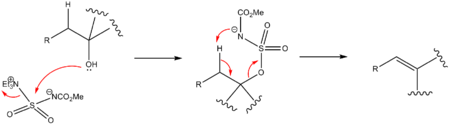
The Burgess reagent is used to convert secondary and tertiary alcohols with an adjacent proton into alkenes. Dehydration of primary alcohols does not work well. The reagent is soluble in common organic solvents and alcohol dehydration takes place with syn elimination through an intramolecular elimination reaction. The Burgess reagent is a carbamate and an inner salt. A general mechanism is shown below.
Preparation
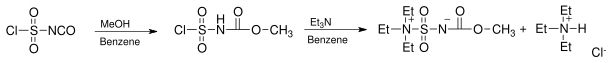
The reagent is prepared from chlorosulfonylisocyanate by reaction with methanol and triethylamine in benzene:[3]
References
- ↑ Atkins, G. M.; Burgess, E. M. (1968). "The reactions of an N-sulfonylamine inner salt". J. Am. Chem. Soc. 90 (17): 4744–4745. doi:10.1021/ja01019a052.
- ↑ Sachin Khapli, Satyajit Dey; Dipakranjan Mal (2001). "Burgess reagent in organic synthesis". J. Indian Inst. Sci. 81: 461–476. Archived from the original on 2004-03-02. https://web.archive.org/web/20040302130947/http://journal.library.iisc.ernet.in/vol200104/paper6/sachin.pdf.
- ↑ Edward M. Burgess; Harold R. Penton Jr.; E. A. Taylor (1973). "Thermal reactions of alkyl N-carbomethoxysulfamate esters". J. Org. Chem. 38 (1): 26–31. doi:10.1021/jo00941a006.
 |
Licensed under CC BY-SA 3.0 | Source: https://handwiki.org/wiki/Chemistry:Burgess_reagent9 views | Status: cached on August 09 2024 09:37:05↧ Download this article as ZWI file
 KSF
KSF