Caesium monoxide
Topic: Chemistry
 From HandWiki - Reading time: 7 min
From HandWiki - Reading time: 7 min
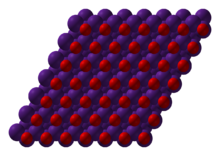 Caesium cations, Cs+ Oxide anions, O2− | |
| Names | |
|---|---|
| IUPAC name
Caesium oxide
| |
| Other names
Cesium oxide (United States )
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| |
| |
| Properties | |
| Cs2O | |
| Molar mass | 281.810 g·mol−1 |
| Appearance | Yellow-orange solid |
| Density | 4.65 g/cm3, solid |
| Melting point | 490 °C (914 °F; 763 K) (under N 2) |
| Reacts to form CsOH | |
| 1534.0·10−6 cm3/mol | |
| Structure | |
| anti-CdCl 2 (hexagonal) | |
| Thermochemistry | |
Heat capacity (C)
|
76.0 J/(K·mol) |
Std molar
entropy (S |
146.9 J/(K·mol) |
Std enthalpy of
formation (ΔfH⦵298) |
−345.8 kJ/mol |
| Hazards | |
| Main hazards | Corrosive |
| GHS pictograms |  
|
| NFPA 704 (fire diamond) | |
| Flash point | non-flammable |
| Related compounds | |
Other anions
|
|
Other cations
|
|
Related compounds
|
Caesium hydroxide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Caesium monoxide or caesium oxide is an chemical compound with the chemical formula Cs
2O. It is the simplest and most common oxide of the caesium. It forms yellow-orange hexagonal crystals.[1]
Uses
Caesium oxide is used in photocathodes to detect infrared signals in devices such as image intensifiers, vacuum photodiodes, photomultipliers, and TV camera tubes[3] L. R. Koller described the first modern photoemissive surface in 1929–1930 as a layer of caesium on a layer of caesium oxide on a layer of silver.[4] It is a good electron emitter; however, its high vapor pressure limits its usefulness.[5]
Reactions
Elemental magnesium reduces caesium oxide to elemental caesium, forming magnesium oxide as a side-product:[6][7]
- Cs
2O + Mg → 2 Cs + MgO
Cs
2O is hygroscopic, forming the corrosive CsOH on contact with water.
References
- ↑ 1.0 1.1 Lide, David R., ed (2006). CRC Handbook of Chemistry and Physics (87th ed.). Boca Raton, FL: CRC Press. pp. 451, 514. ISBN 0-8493-0487-3..
- ↑ Greenwood, Norman N.; Earnshaw, Alan (1984). Chemistry of the Elements. Oxford: Pergamon Press. pp. 97–100. ISBN 978-0-08-022057-4. https://books.google.com/books?id=OezvAAAAMAAJ&q=0-08-022057-6&dq=0-08-022057-6&source=bl&ots=m4tIRxdwSk&sig=XQTTjw5EN9n5z62JB3d0vaUEn0Y&hl=en&sa=X&ei=UoAWUN7-EM6ziQfyxIDoCQ&ved=0CD8Q6AEwBA..
- ↑ Capper, Peter; Elliott, C. T. (2000), Infrared Detectors and Emitters, Springer, p. 14, ISBN 978-0-7923-7206-6, https://books.google.com/books?id=HtgEcjQcgkkC&q=%22cesium+oxide%22+OR+%22caesium+oxide%22&pg=PA14
- ↑ Busch, Kenneth W.; Busch, Marianna A. (1990), Multielement Detection Systems for Spectrochemical Analysis, Wiley-Interscience, p. 12, ISBN 978-0-471-81974-5, https://books.google.com/books?id=9H0W1J-Rku4C&q=%22cesium+oxide%22+OR+%22caesium+oxide%22&pg=PA12
- ↑ Boolchand, Punit, ed. (2000), Insulating and Semiconducting Glasses, World Scientific, p. 855, ISBN 978-981-02-3673-1, Bibcode: 2000isg..book.....B, https://books.google.com/books?id=QK2f4eVh7qgC&q=%22cesium+oxide%22+OR+%22caesium+oxide%22&pg=PA855
- ↑ Turner Jr., Francis M., ed. (1920), The Condensed Chemical Dictionary, New York: Chemical Catalog Co., p. 121, https://books.google.com/books?id=y8y0XE0nsYEC&q=%22cesium+oxide%22+OR+%22caesium+oxide%22&pg=PA121
- ↑ Arora, M.G. (1997), S-Block Elements, New Delhi: Anmol Publications, p. 13, ISBN 978-81-7488-562-3, https://books.google.com/books?id=QR3TCaKaykEC&q=%22Bromine+dioxide%22&pg=PA256
 |
 KSF
KSF
