Calcium pyrophosphate
Topic: Chemistry
 From HandWiki - Reading time: 5 min
From HandWiki - Reading time: 5 min
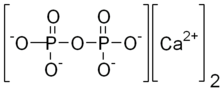
| |
| Names | |
|---|---|
| IUPAC name
Calcium diphosphate
| |
| Other names
Diphosphoric acid, calcium salt (1:2); Dicalcium diphosphate; Dicalcium pyrophosphate
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| MeSH | Calcium+pyrophosphate |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| Ca2O7P2 | |
| Molar mass | 254.053 g/mol |
| Appearance | White powder |
| Density | 3.09 g/cm3 |
| Melting point | 1,353 °C (2,467 °F; 1,626 K) |
| insoluble | |
| Solubility | soluble in HCl, nitric acids |
Refractive index (nD)
|
1.585 |
| Hazards | |
| NFPA 704 (fire diamond) | |
| Flash point | Non-flammable |
| Related compounds | |
Other anions
|
Calcium phosphate |
Other cations
|
Magnesium pyrophosphate Sodium pyrophosphate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Calcium pyrophosphate (Ca2P2O7) is a chemical compound, an insoluble calcium salt containing the pyrophosphate anion. There are a number of forms reported: an anhydrous form, a dihydrate, Ca2P2O7·2H2O and a tetrahydrate, Ca2P2O7·4H2O. Deposition of dihydrate crystals in cartilage are responsible for the severe joint pain in cases of calcium pyrophosphate deposition disease (pseudo gout) whose symptoms are similar to those of gout.[1] Ca2P2O7 is commonly used as a mild abrasive agent in toothpastes,[citation needed] because of its insolubility and nonreactivity toward fluoride.[2]
Preparation
Crystals of the tetrahydrate can be prepared by reacting sodium pyrophosphate, Na4P2O7 with calcium nitrate, Ca(NO3)2, at carefully controlled pH and temperature:[3]
- Na4P2O7(aq)+2 Ca(NO3)2(aq)→ Ca2P2O7·4 H2O + 4 NaNO3
The dihydrate, sometimes termed CPPD, can be formed by the reaction of pyrophosphoric acid with calcium chloride:[citation needed]
- CaCl2 + H4P2O7(aq) → Ca2P2O7·2 H2O + HCl.
The anhydrous forms can be prepared by heating dicalcium phosphate:[citation needed]
- 2 CaHPO4 → Ca2P2O7 + H2O
At 240-500 °C an amorphous phase is formed, heating to 750 °C forms β-Ca2P2O7, heating to 1140 - 1350 °C forms the α-Ca2P2O7.
Structure of anhydrous and hydrated forms
The stable tetrahydrate was originally reported to be rhombohedral but is now believed to be monoclinic. Additionally there is an unstable monoclinic form.[3]
The dihydrate is triclinic, with hydrogen bonding between the two water molecules and hydrogen bonds to the O atoms on the anion.[citation needed] An hexagonal dihydrate has also been reported.[4]
The anhydrous form has 3 polymorphs, α-, β-, and metastable γ[5] (Tα/β=1140ºС[6]). The high temperature form α- is monoclinic (P21/n, a=12.66(1)Å, b=8.542(8)Å, c=5.315(5)Å, Z=4, ρα=2.95 g/cm3), with 8 coordinate calcium, the lower temperature form β- is tetragonal (P41, a=b=6.684Å, c=24.144Å, V=915.40Å3, Z=8, ρβ=3.128 g/cm3), with calcium in four different coordination environments, 2 that are 7 coordinate, one eight and one 9. In both the pyrophosphates are essentially eclipsed.[7][8][9]
References
- ↑ Calcium Pyrophosphate Deposition Disease at eMedicine
- ↑ Klaus Schrödter; Gerhard Bettermann; Thomas Staffel; Friedrich Wahl; Thomas Klein; Thomas Hofmann (2012). "Ullmann's Encyclopedia of Industrial Chemistry". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a19_465.pub3.
- ↑ 3.0 3.1 Christoffersen, Margaret R.; Balic-Zunic, Tonci; Pehrson, Søren; Christoffersen, Jørgen (2000). "Growth and precipitation of a monoclinic calcium pyrophosphate tetrahydrate indicating auto-inhibition at pH7". Journal of Crystal Growth 212 (3–4): 500–506. doi:10.1016/S0022-0248(00)00231-1. Bibcode: 2000JCrGr.212..500C.
- ↑ Mandel, Gretchen S.; Renne, Kathleen M.; Kolbach, Ann M.; Kaplan, Wayne D.; Miller, Jay D.; Mandel, Neil S. (1988). "Calcium pyrophosphate crystal deposition disease: Preparation and characterization of crystals". Journal of Crystal Growth 87 (4): 453–462. doi:10.1016/0022-0248(88)90093-0. Bibcode: 1988JCrGr..87..453M.
- ↑ Parodi, J. A.; Hickok, R. L.; Segelken, W. G.; Cooper, J. R. (1965). "Electronic Paramagnetic Resonance Study of the Thermal Decomposition of Dibasic Calcium Orthophosphate" (in en). Journal of the Electrochemical Society 112 (7): 688. doi:10.1149/1.2423665. Bibcode: 1965JElS..112..688P. https://iopscience.iop.org/article/10.1149/1.2423665.
- ↑ Hill, W L; Reynolds, D S; Hendbicks, S B; Jacob, K D (1945-02-01). "Nutritive Evaluation of Defluorinated Phosphates and Other Phosphorus Supplements. I. Preparation and Properties of the Samples". Journal of AOAC International 28 (1): 105–118. doi:10.1093/jaoac/28.1.105. ISSN 0095-9111. https://doi.org/10.1093/jaoac/28.1.105.
- ↑ Calvo, C. (1968-07-01). "Crystal structure of .alpha.-calcium pyrophosphate". Inorganic Chemistry 7 (7): 1345–1351. doi:10.1021/ic50065a019. ISSN 0020-1669. https://doi.org/10.1021/ic50065a019.
- ↑ Parodi, J. A.; Hickok, R. L.; Segelken, W. G.; Cooper, J. R. (1965). "Electronic Paramagnetic Resonance Study of the Thermal Decomposition of Dibasic Calcium Orthophosphate". Journal of the Electrochemical Society 112 (7): 688. doi:10.1149/1.2423665. Bibcode: 1965JElS..112..688P.
- ↑ Webb, N. C. (1966). "The crystal structure of β-Ca2P2O". Acta Crystallographica 21 (6): 942–948. doi:10.1107/S0365110X66004225.
ja:ピロリン酸カルシウム
 |
 KSF
KSF
