Calone
Topic: Chemistry
 From HandWiki - Reading time: 2 min
From HandWiki - Reading time: 2 min
| |
| Names | |
|---|---|
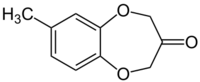
| Preferred IUPAC name
7-Methyl-2,4-dihydro-3H-1,5-benzodioxepin-3-one | |
| Other names
Calone 1951; Watermelon ketone; Methylbenzodioxepinone
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C10H10O3 | |
| Molar mass | 178.187 g·mol−1 |
| Appearance | white crystals, flakes or clumps |
| Odor | distinctive |
| Hazards | |
| Main hazards | irritant |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Calone or methylbenzodioxepinone, trade-named Calone 1951, also known in the industry as "watermelon ketone", was discovered by Pfizer in 1966. It is used to give the olfactory impression of a fresh seashore through the marine and ozone nuances. Calone is similar in structure to brown algae pheromones like ectocarpene and is also distantly related in structure to the benzodiazepine class of sedatives.[1]
Calone is an unusual chemical compound which has an intense "sea-breeze" note with slight floral and fruit overtones. It has been used as a scent component since the 1980s for its watery, fresh, ozone accords, and as a more dominant note in several perfumes of the marine trend, beginning in the 1990s. In 2014, Plummer et al. reported the synthesis and fragrance properties of several related aliphatic analogues. [2] Firmenich later releasing CASCALONE®, A sweet, watery version of CALONE® with a transparent floral signature[3]
References
- ↑ Yudov, Matvey. "Calone: The Air of the 1990s ~ Raw Materials ~ Fragrantica". https://www.fragrantica.com/news/Calone-The-Air-of-the-1990s-8150.html.
- ↑ C. M. Plummer, R. Gericke, P. Kraft, A. Raynor, J. Froese, T. Hudlicky, T. J. Rook, O. A. H. Jones and H. M. Hϋgel (4 Dec 2014). "Synthesis of Saturated Benzodioxepinone Analogues: Insight into the Importance of the Aromatic Ring Binding Motif for Marine Odorants". Eur. J. Org. Chem. 2015 (3): 486–495. doi:10.1002/ejoc.201403142.
- ↑ "CASCALONE®" (in en). https://www.firmenich.com/product/cascaloner-pe-920000-0.
External links
 |
 KSF
KSF