Copper (64Cu) oxodotreotide
Topic: Chemistry
 From HandWiki - Reading time: 6 min
From HandWiki - Reading time: 6 min
 | |
| Clinical data | |
|---|---|
| Trade names | Detectnet |
| License data | |
| Routes of administration | Intravenous |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| UNII | |
| KEGG | |
| Chemical and physical data | |
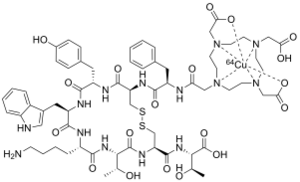
| Formula | C65H88CuN14O19S2 |
| Molar mass | 1497.16 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Copper (64Cu) oxodotreotide or Copper Cu 64 dotatate, sold under the brand name Detectnet, is a radioactive diagnostic agent indicated for use with positron emission tomography (PET) for localization of somatostatin receptor positive neuroendocrine tumors (NETs) in adults.[2]
Common side effects include nausea, vomiting and flushing.[3]
It was approved for medical use in the United States in September 2020.[2][3]
History
The U.S. Food and Drug Administration (FDA) approved copper 64Cu dotatate based on data from two trials that evaluated 175 adults.[4]
Trial 1 evaluated adults, some of whom had known or suspected NETs and some of whom were healthy volunteers.[4] The trial was conducted at one site in the United States (Houston, TX).[4] Both groups received copper 64Cu dotatate and underwent PET scan imaging.[4]
Trial 2 data came from the literature-reported trial of 112 adults, all of whom had history of NETs and underwent PET scan imaging with copper 64Cu dotatate.[4] The trial was conducted at one site in Denmark.[4] In both trials, copper 64Cu dotatate images were compared to either biopsy results or other images taken by different techniques to detect the sites of a tumor.[4] The images were read as either positive or negative for presence of NETs by three independent image readers who did not know participant clinical information.[4]
See also
References
- ↑ "Detectnet- copper cu 64 dotatate injection, solution". 14 September 2020. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=f898c8a7-beb7-4318-a8ea-6f058d69342b.
- ↑ 2.0 2.1 "FDA approval letter". 3 September 2020. https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2020/213227Orig1s000ltr.pdf.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ 3.0 3.1 "RadioMedix and Curium Announce FDA Approval of Detectnet (copper Cu 64 dotatate injection) in the U.S." (Press release). Curium. 8 September 2020. Retrieved 9 September 2020 – via GlobeNewswire.
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 4.6 4.7 "Drug Trials Snapshots: Detectnet". 3 September 2020. https://www.fda.gov/drugs/drug-approvals-and-databases/drug-trials-snapshots-detectnet.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
External links
- "copper Cu 64 dotatate injection safety data sheet". Curium US LLC. 15 March 2020. https://www.curiumpharma.com/wp-content/uploads/2020/08/SDS-US-copper-Cu-64-dotatate-injection_.pdf.
 |
 KSF
KSF