Copper salicylate
Topic: Chemistry
 From HandWiki - Reading time: 1 min
From HandWiki - Reading time: 1 min
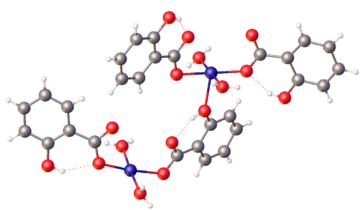
Structure of a hydrated copper(II) salicylate.[1] Color code: red = O, blue = Cu, gray = C, white = H.
Copper salicylate describes a range of compounds containing copper(II) and salicylate. Many compounds are known. They are generally blue. Simple species include:
- Cu
2(
2O
2C
6H
4OH)
4(H
2O)
2 · L) (L = diverse solvent.[4]
Many copper(II) salicylates are known with amines and N-hetrerocyclic ligands. None appear to have commercial applications.
References
- ↑ Lutz, Martin; Kroon-Batenburg, Loes M. J. (2018). "Order-Disorder in Diaquobis(salicylato)copper(II) Revisited". Croatica Chemica Acta 91 (2). doi:10.5562/cca3362.
- ↑ Jagner, S.; Hazell, R. G.; Larsen, K. P. (1976). "The crystal structure of diaquabis(salicylato)copper(II), Cu[C6H4(OH)COO]2(H2O)2". Acta Crystallographica Section B Structural Crystallography and Crystal Chemistry 32 (2): 548–554. doi:10.1107/S0567740876003397. Bibcode: 1976AcCrB..32..548J.
- ↑ Hanic, F.; Michalov, J. (1960). "Die Kristallstruktur von Kupfersalicylat-Tetrahydrat Cu(OH.C6H4.COO)2.4H2O". Acta Crystallographica 13 (4): 299–302. doi:10.1107/S0365110X60000753. Bibcode: 1960AcCry..13..299H.
- ↑ V.V.Gorinchoy, Yu.A.Simonov, S.G.Shova, Y.N.Szafranski, K.I.Turta (2009). Zh.Strukt.Khim.(Russ.)(J.Struct.Chem.) 50: 1196.
 |
Licensed under CC BY-SA 3.0 | Source: https://handwiki.org/wiki/Chemistry:Copper_salicylate6 views | Status: cached on September 21 2024 14:59:15↧ Download this article as ZWI file
 KSF
KSF