Corrosion inhibitors for petroleum industry
Topic: Chemistry
 From HandWiki - Reading time: 5 min
From HandWiki - Reading time: 5 min
Corrosion inhibitors for the petroleum industry are substances used in the oil industry to protect equipment and pipelines against corrosion. Corrosion is a daily problem in the oil industry due to the presence in crude oil of water contaminated with salts, gases and other corrosive contaminants in the production process.
Corrosion inhibitors can be classified according to their chemical composition as either organic inhibitors or inorganic inhibitors. They can also be classified by the way they act as anodic or cathodic inhibitors. Cathodic inhibitors act as catalysts to slow down corrosion, while anodic inhibitors protect metal surfaces by acting as physical barriers.[1]
Corrosion inhibitors are used in different processes in the petroleum industry, such as well drilling, pipeline transportation in and out of the refinery, fuel production, in water treatment systems and in oil and gas storage.[2]
Corrosion inhibitors come in a variety of chemical families, some of which are used in the oil industry. Acid inhibitors, sulfide inhibitors, oxygen inhibitors, and corrosion-promoting microorganism inhibitors are a few types of corrosion inhibitors used in the oil industry. They can also be classified by their chemical composition into organic and inorganic corrosion inhibitors.[3]
Some chemical families of corrosion inhibitors used in the oil industry
There are different chemical families of corrosion inhibitors used in the oil industry, among them are the following:
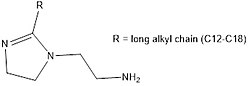
Fatty imidazolines: These are imidazole-based compounds, with long unsaturated chains, derived mainly from oleic acid. They are mainly used to prevent acid corrosion of carbon steel. They form a filmic layer inside pipes and equipment, reducing the interactions between the fluid and the metal (Figure 1).[4]
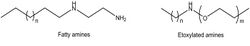
Fatty amines: These corrosion inhibitors are nitrogenous organic compounds containing an amino group and a long-chain alkyl group (more than 12 carbon atoms). They act as cathodic inhibitors and form a protective layer on the metal surface. They are also effective against corrosion caused by carbon dioxide. (CO2) and hydrogen sulfide (H2S). Also, ethoxylated amines are used for the same purpose (Figure 2).[5]
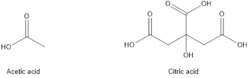
Organic acids: Organic acids such as acetic acid, formic acid and citric acid are used as corrosion inhibitors. These acids react with metal ions to form insoluble compounds that protect the metal surface. These inhibitors are often used in combination with other corrosion inhibitors and techniques, such as cathodic protection and coatings, to provide comprehensive corrosion protection. They are effective at controlling corrosion caused by CO2 and H2S, which are commonly found in petroleum reservoirs and can be highly corrosive. (Figure 3).[6]
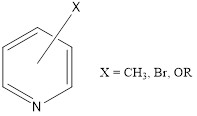
Pyridines: Some studies have shown that certain pyridines can inhibit corrosion caused by the presence of acid gases, such as CO2 and H2S, which are common in the oil industry. Pyridine and its derivatives have been shown to be effective inhibitors for a wide range of metals, such as carbon steel, stainless steel, and copper alloys. They act by adsorbing to the metal surface and forming a protective film, which can be physical or chemical in nature. Pyridine and its derivatives are also effective in inhibiting localized corrosion, such as pitting and crevice corrosion (Figure 4).[7]
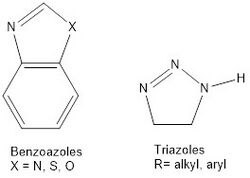
Azoles: Azoles, such as triazole and benzotriazole, oxazole and benzoxales, and thioazoles and benzothioazoles are organic compounds used as corrosion inhibitors in the petroleum industry. They act as anodic inhibitors and form a protective layer on the metal surface (Figure 5).[8]
Polymers: Polymers are large molecules with high molecular weight used in the petroleum industry as corrosion inhibitors. Some polymers can adsorb onto the metal surface and form a protective coating. They can also be used as dispersants to prevent the formation of corrosive deposits. Some examples are:
Aminated polymers: These polymers are used for corrosion protection of metal surfaces in the oil industry. They are highly effective in preventing salt water and H2S corrosion.
Acrylic polymers: These polymers are used as corrosion inhibitors in the industry due to their good compatibility with oil and drilling fluids. They are effective against corrosion caused by the presence of hydrochloric acid (HCl) in drilling fluids.
Maleate polymers: These polymers are used as corrosion inhibitors in the industry due to their good adsorption capacity on metal surfaces and their high solubility in oil and drilling fluids. They are effective against corrosion caused by the presence of H2S in drilling fluids. (Figure 6).[9]

Other organic products used as corrosion inhibitors in oil industry are nitriles, amides, oximes, ureas and thioureas, phosphonate salts. Inorganic inhibitors such as lanthanides, molybdates, silicates, boric and phosphoric acid, and the combination of nitrates and nitrites are also widely employed,[10] as well as more environmentally friendly inhibitors such as some biomass wastes, amino acids, and ionic liquids.[11]
These inhibitors work by forming a protective layer on the surface of the metal, which prevents acids and other corrosive chemicals from coming into direct contact with the metal. It is critical to choose the right corrosion inhibitor based on the type of corrosion present, environmental conditions, temperature, pressure and type of metal to be protected, and to ensure that the right amount is applied for maximum protection. Periodic corrosion rate monitoring should also be performed to confirm that our inhibitor is working properly.
References
- ↑ Sastri, V. S. (1998). Corrosion Inhibitors. Principles and Applications. Wiley. ISBN 9780471976080. https://www.abebooks.com/9780471976080/Corrosion-Inhibitors-Principles-Applications-Sastri-0471976083/plp. Retrieved 2023-04-17.
- ↑ Popova, A.; Christov, M.; Zwetanova, A. (May 2007). "Effect of the molecular structure on the inhibitor properties of azoles on mild steel corrosion in 1M hydrochloric acid" (in en). Corrosion Science 49 (5): 2131–2143. doi:10.1016/j.corsci.2006.10.021. https://linkinghub.elsevier.com/retrieve/pii/S0010938X06003489. Retrieved 2023-04-17.
- ↑ Martínez Palou, Rafael; Likhanova, Natalya V. (2023), "Application of ionic liquids as Corrosion Inhibitors in the Oil Industry", Applications of Ionic Liquids in the Oil Industry: Towards A Sustainable Industry (BENTHAM SCIENCE PUBLISHERS): pp. 94–121, doi:10.2174/9789815079579123010008, ISBN 978-981-5079-57-9, OCLC 1370200690, http://dx.doi.org/10.2174/9789815079579123010008, retrieved 2023-10-01
- ↑ Olivares-Xometl, O.; Likhanova, N. V.; Martínez-Palou, R.; Domínguez-Aguilar, M. A. (January 2009). "Electrochemistry and XPS study of an imidazoline as corrosion inhibitor of mild steel in an acidic environment" (in en). Materials and Corrosion 60 (1): 14–21. doi:10.1002/maco.200805044. https://onlinelibrary.wiley.com/doi/10.1002/maco.200805044. Retrieved 2023-04-17.
- ↑ Cruz, J; Martı́nez, R; Genesca, J; Garcı́a-Ochoa, E (May 2004). "Experimental and theoretical study of 1-(2-ethylamino)-2-methylimidazoline as an inhibitor of carbon steel corrosion in acid media" (in en). Journal of Electroanalytical Chemistry 566 (1): 111–121. doi:10.1016/j.jelechem.2003.11.018. https://linkinghub.elsevier.com/retrieve/pii/S0022072803007228. Retrieved 2023-04-17.
- ↑ Martínez-Palou, R.; Rivera, J.; Zepeda, L. G.; Rodríguez, A. N.; Hernández, M. A.; Marín-Cruz, J.; Estrada, A. (May 2004). "Evaluation of Corrosion Inhibitors Synthesized from Fatty Acids and Fatty Alcohols Isolated from Sugar Cane Wax" (in en). Corrosion 60 (5): 465–470. doi:10.5006/1.3299242. ISSN 0010-9312. http://corrosionjournal.org/doi/10.5006/1.3299242. Retrieved 2023-04-17.
- ↑ Abd El-Maksoud, S.A.; Fouda, A.S. (September 2005). "Some pyridine derivatives as corrosion inhibitors for carbon steel in acidic medium" (in en). Materials Chemistry and Physics 93 (1): 84–90. doi:10.1016/j.matchemphys.2005.02.020. https://linkinghub.elsevier.com/retrieve/pii/S0254058405001409. Retrieved 2023-04-17.
- ↑ Likhanova, Natalya V.; Martínez-Palou, Rafael; Veloz, M. Aurora; Matías, Diana J.; Reyes-Cruz, Victor E.; Höpfl, Herbert; Olivares, Octavio (January 2007). "Microwave-assisted synthesis of 2-(2-pyridyl)azoles. Study of their corrosion inhibiting properties" (in en). Journal of Heterocyclic Chemistry 44 (1): 145–153. doi:10.1002/jhet.5570440123. https://onlinelibrary.wiley.com/doi/10.1002/jhet.5570440123. Retrieved 2023-04-17.
- ↑ Likhanova, Natalya V.; López-Prados, Nallely; Guzmán-Lucero, Diego; Olivares-Xometl, Octavio; Lijanova, Irina V.; Arellanes-Lozada, Paulina; Arriola-Morales, Janette (2022-04-18). "Some polymeric imidazolates from alkylimidazolium as corrosion inhibitors of API 5L X52 steel in production water" (in en). Journal of Adhesion Science and Technology 36 (8): 845–874. doi:10.1080/01694243.2021.1939600. ISSN 0169-4243. https://www.tandfonline.com/doi/full/10.1080/01694243.2021.1939600. Retrieved 2023-04-17.
- ↑ Bethencourt, M.; Botana, F.J.; Calvino, J.J.; Marcos, M.; RodrÍguez-Chacón, M.A. (November 1998). "Lanthanide compounds as environmentally-friendly corrosion inhibitors of aluminium alloys: a review" (in en). Corrosion Science 40 (11): 1803–1819. doi:10.1016/S0010-938X(98)00077-8. https://linkinghub.elsevier.com/retrieve/pii/S0010938X98000778. Retrieved 2023-04-17.
- ↑ Martinez, Rafael; Olivares-Xomelt, Octavio; V. Likhanov, Natalya (2014-02-20). "Environmentally Friendly Corrosion Inhibitors". in Aliofkhazraei, M. (in en). Developments in Corrosion Protection. InTech. doi:10.5772/57252. ISBN 978-953-51-1223-5. http://www.intechopen.com/books/developments-in-corrosion-protection/environmentally-friendly-corrosion-inhibitors. Retrieved 2023-04-17.
 KSF
KSF