Coutaric acid
Topic: Chemistry
 From HandWiki - Reading time: 2 min
From HandWiki - Reading time: 2 min
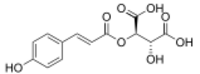
| |
| Names | |
|---|---|
| Preferred IUPAC name
(2R,3R)-2-Hydroxy-3-{[(2E)-3-(4-hydroxyphenyl)prop-2-enoyl]oxy}butanedioic acid | |
| Other names
trans-coutaric acid
cis-coutaric acid trans-p-Coumaroyltartaric acid cis-p-coumaroyl-(+)-tartaric acid trans-p-coumaroyl-(+)-tartaric acid cis-Coumaroyl tartaric acidbr>trans-Coumaroyl tartaric acid | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C13H12O8 | |
| Molar mass | 296.231 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Coutaric acid is a hydroxycinnamoyltartaric acid found in wine, pomace[1] and grape.[2] It is an ester formed from coumaric acid and tartaric acid.
References
- ↑ Maier, T.; Sanzenbacher, S.; Kammerer, D. R.; Berardini, N.; Conrad, J. R.; Beifuss, U.; Carle, R.; Schieber, A. (2006). "Isolation of hydroxycinnamoyltartaric acids from grape pomace by high-speed counter-current chromatography". Journal of Chromatography A 1128 (1–2): 61–67. doi:10.1016/j.chroma.2006.06.082. PMID 16860334.
- ↑ Singleton, V. L.; Zaya, J.; Trousdale, E. K. (1986). "Caftaric and coutaric acids in fruit of Vitis". Phytochemistry 25 (9): 2127. doi:10.1016/0031-9422(86)80078-4.
 |
Licensed under CC BY-SA 3.0 | Source: https://handwiki.org/wiki/Chemistry:Coutaric_acid7 views | ↧ Download this article as ZWI file
 KSF
KSF