Crocin
Topic: Chemistry
 From HandWiki - Reading time: 7 min
From HandWiki - Reading time: 7 min
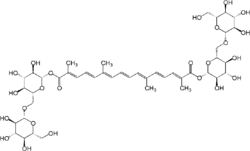
| |
| Names | |
|---|---|
| IUPAC name
Bis[β-D-glucopyranosyl-(1→6)-β-D-glucopyranosyl] 8,8′-diapocarotene-8,8′-dioate
| |
| Systematic IUPAC name
Bis[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-({[(2R,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}methyl)oxan-2-yl] (2E,4E,6E,8E,10E,12E,14E)-2,6,11,15-tetramethylhexadeca-2,4,6,8,10,12,14-heptaenedioate | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C44H64O24 | |
| Molar mass | 976.972 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Crocin is a carotenoid chemical compound that is found in the flowers of crocus and gardenia.[1] Its oxygen content also chemically makes it a xanthene. Crocin is the chemical primarily responsible for the color of saffron.
Chemically, crocin is the diester formed from the disaccharide gentiobiose and the dicarboxylic acid crocetin. When isolated as a pure chemical compound, it has a deep red color and forms crystals with a melting point of 186 °C. When dissolved in water, it forms an orange solution.
The term crocins may also refer to members of a series of related hydrophilic carotenoids that are either monoglycosyl or diglycosyl polyene esters of crocetin.[2] The crocin underlying saffron's aroma is α-crocin (a carotenoid pigment that may compose more than 10% of dry saffron's mass): trans-crocetin di-(β-D-gentiobiosyl) ester; it bears the systematic (IUPAC) name 8,8-diapo-8,8-carotenoic acid.[2]: 20
The major active component of saffron is the yellow pigment crocin 2 (three other derivatives with different glycosylations are known) containing a gentiobiose (disaccharide) group at each end of the molecule. The five major biologically active components of saffron, namely the four crocins and crocetin, can be measured with HPLC-UV.[3]
Research
Absorption
Crocin ingested orally is hydrolised to crocetin in the gut which is absorbed across the intestinal barrier, and that crocetin can permeate the blood–brain barrier.[4][5]
Antioxidant
Crocin has been shown to be an antioxidant,[6][7] and neural protective agent.[8][9] Crocin can reduce oxidative stress and ROS (Reactive Oxygen Species) through enhancement of gene expression of Nrf2, HO-1, and anti-oxidant enzymes, such as CAT, GSH, and SOD.[10][2][7]
Neuroprotective
Crocin and its derivative crocetin may counteract oxidative stress, mitochondrial dysfunction and neuroinflammation, which are closely linked to initiation and progression of major brain pathologies such as Alzheimer's and Parkinson's disease.[11]
In an animal model of malathion-induced Parkinson's disease, crocin reduced the neurotoxic effect of malathion by its anti-apoptotic activity and it regulated the expression of proteins involved in Parkinson's disease pathogenesis.[12]
Crocins can suppress the active forms of GSK3β and ERK1/2 kinases, significantly reducing tau phosphorylation, thus suppressing key molecular pathways of Alzheimer's disease pathogenesis.[13]
Mood
Crocin displays possible antidepressant properties in mice[14] and humans.[15][16][17]
Cancer
Crocin has also shown antiproliferative action against cancer cells in vitro.[18][19][20] and in vivo .[21]
Crocin through the PI3K/AKT/mTOR, MAPK, VEGF, Wnt/β-catenin, and JAK-STAT suppression has antiproliferative properties. Also, the Nrf2 and p53 signaling pathway activation may be effective in the antiproliferative effect of crocin.[22]
Behavior
Aphrodisiac properties of crocin in male rats has been observed at very high doses.[23]
Retinal diseases
Emerging evidence highlights the cytoprotective, antioxidative, and anti-inflammatory potential of crocin in retinal tissue, which positions it as a promising candidate for enhancing vision and eye health. Nevertheless, it's important to note that the majority of research has primarily focused on animal models, and there remains a shortage of robust clinical data to firmly establish the benefits of crocin in addressing eye health and related diseases.[24]
References
- ↑ "Chemical Information". sun.ars-grin.gov. http://sun.ars-grin.gov:8080/npgspub/xsql/duke/chemdisp.xsql?chemical=CROCIN.
- ↑ 2.0 2.1 2.2 "Cancer chemopreventive and tumoricidal properties of saffron (Crocus sativus L.)". Experimental Biology and Medicine 227 (1): 20–25. January 2002. doi:10.1177/153537020222700104. PMID 11788779.
- ↑ "Simultaneous quantification of five major biologically active ingredients of saffron by high-performance liquid chromatography". Journal of Chromatography A 849 (2): 349–355. July 1999. doi:10.1016/S0021-9673(99)00600-7. PMID 10457433.
- ↑ "Chapter 1 - Biochemistry and metabolism∗" (in en). Chapter 1 - Biochemistry and metabolism. Academic Press. 2021-01-01. 1–40. doi:10.1016/b978-0-12-821219-6.00001-4. ISBN 978-0-12-821219-6. https://www.sciencedirect.com/science/article/pii/B9780128212196000014. Retrieved 2022-08-20.
- ↑ "Intestinal formation of trans-crocetin from saffron extract (Crocus sativus L.) and in vitro permeation through intestinal and blood brain barrier". Phytomedicine 22 (1): 36–44. January 2015. doi:10.1016/j.phymed.2014.10.009. PMID 25636868.
- ↑ "Inhibitory activity on amyloid-beta aggregation and antioxidant properties of Crocus sativus stigmas extract and its crocin constituents". Journal of Agricultural and Food Chemistry 54 (23): 8762–8768. November 2006. doi:10.1021/jf061932a. PMID 17090119.
- ↑ 7.0 7.1 Akhtari K; Hassanzadeh K; Fakhraei B; Fakhraei N; Hassanzadeh H; Zarei S A (2013). "A density functional theory study of the reactivity descriptors and antioxidant behavior of Crocin". Computational and Theoretical Chemistry 1013: 123–129. doi:10.1016/j.comptc.2013.03.015.
- ↑ "Protective effects of carotenoids from saffron on neuronal injury in vitro and in vivo". Biochimica et Biophysica Acta (BBA) - General Subjects 1770 (4): 578–584. April 2007. doi:10.1016/j.bbagen.2006.11.012. PMID 17215084.
- ↑ "Effects of crocin on reperfusion-induced oxidative/nitrative injury to cerebral microvessels after global cerebral ischemia". Brain Research 1138: 86–94. March 2007. doi:10.1016/j.brainres.2006.12.064. PMID 17274961.
- ↑ "Interaction of saffron and its constituents with Nrf2 signaling pathway: A review". Iranian Journal of Basic Medical Sciences 25 (7): 789–798. July 2022. doi:10.22038/ijbms.2022.61986.13719. ISSN 2008-3866. PMID 36033950.
- ↑ "Redox modulation of stress resilience by Crocus sativus L. for potential neuroprotective and anti-neuroinflammatory applications in brain disorders: From molecular basis to therapy". Mechanisms of Ageing and Development 205: 111686. July 2022. doi:10.1016/j.mad.2022.111686. PMID 35609733.
- ↑ "Crocin Protects Malathion-Induced Striatal Biochemical Deficits by Inhibiting Apoptosis and Increasing α-Synuclein in Rats' Striatum". Journal of Molecular Neuroscience 72 (5): 983–993. May 2022. doi:10.1007/s12031-022-01990-3. PMID 35274200.
- ↑ "The Crocus sativus Compounds trans-Crocin 4 and trans-Crocetin Modulate the Amyloidogenic Pathway and Tau Misprocessing in Alzheimer Disease Neuronal Cell Culture Models". Frontiers in Neuroscience 13: 249. 2019-03-26. doi:10.3389/fnins.2019.00249. PMID 30971876.
- ↑ "Effect of Crocus sativus L. (saffron) stigma and its constituents, crocin and safranal, on morphine withdrawal syndrome in mice". Phytotherapy Research 24 (5): 726–730. May 2010. doi:10.1002/ptr.3011. PMID 19827024.
- ↑ "Comparison of Crocus sativus L. and imipramine in the treatment of mild to moderate depression: a pilot double-blind randomized trial [ISRCTN45683816"]. BMC Complementary and Alternative Medicine 4: 12. September 2004. doi:10.1186/1472-6882-4-12. PMID 15341662.
- ↑ "The effects of crocin on the symptoms of depression in subjects with metabolic syndrome". Advances in Clinical and Experimental Medicine 26 (6): 925–930. September 2017. doi:10.17219/acem/62891. PMID 29068592.
- ↑ "Anti-Depressant Properties of Crocin Molecules in Saffron". Molecules 27 (7): 2076. March 2022. doi:10.3390/molecules27072076. PMID 35408474.
- ↑ "Crocin, safranal and picrocrocin from saffron (Crocus sativus L.) inhibit the growth of human cancer cells in vitro". Cancer Letters 100 (1–2): 23–30. February 1996. doi:10.1016/0304-3835(95)04067-6. PMID 8620447.
- ↑ "Inhibition of breast cancer cell proliferation by style constituents of different Crocus species". Anticancer Research 27 (1A): 357–362. 2007. PMID 17352254.
- ↑ "[In vitro evaluation of the chemopreventive potential of saffron]". Revista de Investigacion Clinica 54 (5): 430–436. 2002. PMID 12587418.
- ↑ "Crocin Inhibits Angiogenesis and Metastasis in Colon Cancer via TNF-α/NF-kB/VEGF Pathways". Cells 11 (9): 1502. April 2022. doi:10.3390/cells11091502. PMID 35563808.
- ↑ "Crocin molecular signaling pathways at a glance: A comprehensive review". Phytotherapy Research 36 (10): 3859–3884. August 2022. doi:10.1002/ptr.7583. PMID 35989419.
- ↑ "The effect of saffron, Crocus sativus stigma, extract and its constituents, safranal and crocin on sexual behaviors in normal male rats". Phytomedicine 15 (6–7): 491–495. June 2008. doi:10.1016/j.phymed.2007.09.020. PMID 17962007.
- ↑ Heydari, Mojtaba; Zare, Mousa; Badie, Mohammad Reza; Watson, Ronald Ross; Talebnejad, Mohammad Reza; Afarid, Mehrdad (April 2023). "Crocin as a vision supplement". Clinical & Experimental Optometry 106 (3): 249–256. doi:10.1080/08164622.2022.2039554. ISSN 1444-0938. https://pubmed.ncbi.nlm.nih.gov/35231199/.
Bibliography
- NCBI (2022). "CID 5281233, Crocin". Bethesda MD: National Library of Medicine. https://pubchem.ncbi.nlm.nih.gov/compound/Crocin.
External links
 |
 KSF
KSF