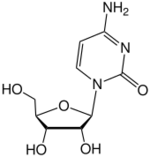
Cytidine
Topic: Chemistry
 From HandWiki - Reading time: 7 min
From HandWiki - Reading time: 7 min
| |
| |
| Names | |
|---|---|
| IUPAC name
Cytidine
| |
| Systematic IUPAC name
4-Amino-1-[(2R,3R,4S,5R)-3,4-dihydroxy-5-(hydroxymethyl)oxolan-2-yl]pyrimidin-2(1H)-one | |
| Other names
4-Amino-1-β-D-ribofuranosyl-2(1H)-pyrimidinone[1]
4-Amino-1-[3,4-dihydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl]pyrimidin-2-one | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| KEGG | |
| MeSH | Cytidine |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C9H13N3O5 | |
| Molar mass | 243.217 |
| Appearance | white, crystalline powder[2] |
| Melting point | 230 °C (decomposes)[1] |
| -123.7·10−6 cm3/mol | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Cytidine (symbol C or Cyd) is a nucleoside molecule that is formed when cytosine is attached to a ribose ring (also known as a ribofuranose) via a β-N1-glycosidic bond. Cytidine is a component of RNA. It is a white water-soluble solid.[2] which is only slightly soluble in ethanol.[1]
Dietary sources
Dietary sources of cytidine include foods with high RNA (ribonucleic acid) content,[3] such as organ meats, brewer's yeast, as well as pyrimidine-rich foods such as beer. During digestion, RNA-rich foods are broken-down into ribosyl pyrimidines (cytidine and uridine), which are absorbed intact.[3] In humans, dietary cytidine is converted into uridine,[4] which is probably the compound behind cytidine's metabolic effects.
Cytidine analogues
A variety of cytidine analogues are known, some with potentially useful pharmacology. For example, KP-1461 is an anti-HIV agent that works as a viral mutagen,[5] and zebularine exists in E. coli and is being examined for chemotherapy. Low doses of azacitidine and its analog decitabine have shown results against cancer through epigenetic demethylation.[6]
Biological actions
In addition to its role as a pyrimidine component of RNA, cytidine has been found to control neuronal-glial glutamate cycling, with supplementation decreasing midfrontal/cerebral glutamate/glutamine levels.[7] As such, cytidine has generated interest as a potential glutamatergic antidepressant drug.[7]
Related compounds
- Deoxycytidine is cytosine attached to a deoxyribose.
Properties
References
- ↑ 1.0 1.1 1.2 William M. Haynes (2016). CRC Handbook of Chemistry and Physics (97th ed.). Boca Raton: CRC Press. pp. 3–140. ISBN 978-1-4987-5429-3. https://books.google.com/books?id=VVezDAAAQBAJ.
- ↑ 2.0 2.1 Robert A. Lewis, Michael D. Larrañaga, Richard J. Lewis Sr. (2016). Hawley's Condensed Chemical Dictionary (16th ed.). Hoboken, New Jersey: John Wiley & Sons, Inc.. p. 688. ISBN 978-1-118-13515-0.
- ↑ 3.0 3.1 Jonas DA; Elmadfa I; Engel KH et al. (2001). "Safety considerations of DNA in food". Ann Nutr Metab 45 (6): 235–54. doi:10.1159/000046734. PMID 11786646. http://content.karger.com/produktedb/produkte.asp?typ=fulltext&file=anm45235.
- ↑ "Effect of oral CDP-choline on plasma choline and uridine levels in humans". Biochem. Pharmacol. 60 (7): 989–92. Oct 2000. doi:10.1016/S0006-2952(00)00436-6. PMID 10974208.
- ↑ John S. James. "New Kind of Antiretroviral, KP-1461". AIDS Treatment News. http://www.aidsnews.org/2007/10/kp-1461.html.
- ↑ "Scientists reprogram cancer cells with low doses of epigenetic drugs". Medical XPress. March 22, 2012. http://medicalxpress.com/news/2012-03-scientists-reprogram-cancer-cells-doses.html.
- ↑ 7.0 7.1 Machado-Vieira, Rodrigo; Salvadore, Giacomo; DiazGranados, Nancy; Ibrahim, Lobna; Latov, David; Wheeler-Castillo, Cristina; Baumann, Jacqueline; Henter, Ioline D. et al. (2010). "New Therapeutic Targets for Mood Disorders". The Scientific World Journal 10: 713–726. doi:10.1100/tsw.2010.65. ISSN 1537-744X. PMID 20419280.
External links
 |
 KSF
KSF