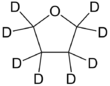
Deuterated THF
Topic: Chemistry
 From HandWiki - Reading time: 4 min
From HandWiki - Reading time: 4 min
Short description: Chemical compound
| |||
| |||
| Identifiers | |||
|---|---|---|---|
3D model (JSmol)
|
|||
| 111854 | |||
| ChemSpider | |||
| EC Number |
| ||
PubChem CID
|
|||
| UN number | 2056 | ||
| |||
| |||
| Properties | |||
| C4D8O | |||
| Molar mass | 80.1550 g mol−1 | ||
| Appearance | Colourless liquid | ||
| Density | 985 mg cm−3 | ||
| Melting point | −106 °C (−159 °F; 167 K) | ||
| Boiling point | 65 to 66 °C (149 to 151 °F; 338 to 339 K) | ||
| Hazards | |||
| GHS pictograms |  
| ||
| GHS Signal word | DANGER | ||
| H225, H319, H335 | |||
| P210, P261, P305+351+338 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | −17 °C (1 °F; 256 K) | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Tracking categories (test):
Deuterated tetrahydrofuran (d8-THF) is a colourless, organic liquid at standard temperature and pressure.[1] This heterocyclic compound has the chemical formula C4D8O, and is an isotopologue of tetrahydrofuran.[2] Deuterated THF is used as a solvent in NMR spectroscopy, though its expense can often be prohibitive.[citation needed]
References
- ↑ Andersson, O.; Suga, H. (1996-01-01). "Thermal conductivity of normal and deuterated tetrahydrofuran clathrate hydrates" (in en). Journal of Physics and Chemistry of Solids 57 (1): 125–132. doi:10.1016/0022-3697(95)00157-3. ISSN 0022-3697. https://dx.doi.org/10.1016/0022-3697%2895%2900157-3.
- ↑ David, W. I. F.; Ibberson, R. M. (1992-02-15). "A reinvestigation of the structure of tetrahydrofuran by high-resolution neutron powder diffraction" (in en). Acta Crystallographica Section C: Crystal Structure Communications 48 (2): 301–303. doi:10.1107/S0108270191008582. ISSN 0108-2701. http://scripts.iucr.org/cgi-bin/paper?S0108270191008582.
 |
Licensed under CC BY-SA 3.0 | Source: https://handwiki.org/wiki/Chemistry:Deuterated_THF3 views | ↧ Download this article as ZWI file
 KSF
KSF