Dysprosium titanate
Topic: Chemistry
 From HandWiki - Reading time: 7 min
From HandWiki - Reading time: 7 min
| |
| Names | |
|---|---|
| IUPAC name
Dysprosium titanate
| |
| Identifiers | |
3D model (JSmol)
|
|
| |
| |
| Properties | |
| Dy2O7Ti2 | |
| Molar mass | 532.727 g·mol−1 |
| Density | 6.8 g/cm3[1] |
| Structure[1] | |
| Pyrochlore | |
| Fd3m, cF88, No. 227 | |
a = 1.0136 nm
| |
Formula units (Z)
|
8 |
| Related compounds | |
Other cations
|
Holmium titanate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Dysprosium titanate (Dy2Ti2O7 or Dy2TiO5) is an inorganic compound, specifically a ceramic of the titanate family. Two common phases of this compound exist with differing properties: Dy2Ti2O7 and Dy2TiO5. Dysprosium titanate is commonly used throughout the nuclear industry in nuclear control rods and as a host for nuclear waste.[2][3]
History
Dysprosium titanate was one of the first materials that was discovered to be a spin ice, along with holmium titanate (Ho2Ti2O7), in 1997.[4] The existence of these materials was predicted by Linus Pauling in 1935, but neutron scattering experiments confirmed their existence as holmium titanate satisfied the model.[5]
Since its discovery as a spin ice, dysprosium titanate has continued to be a focus of research because the magnetic frustration that results from its pyrochlore lattice. In 2009, quasiparticles resembling magnetic monopoles were observed at low temperature and high magnetic field through neutron-scattering experiments.[6] The study demonstrated the existence of Dirac strings in dysprosium titanate and the presence of monopole characteristics at low temperatures.[7]
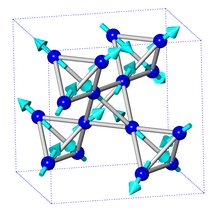
Structure
The Dy2Ti2O7 phase exhibits a cubic pyrochlore structure where the Dy3+ ions form a network of corner-sharing tetrahedra.[4][8] It is notable for its ability to withstand structural change in the presence of radiation from high energy ions.[2]
Dy2Ti2O7 can be "stuffed" by adding additional lanthanide atoms into the pyrochlore to generate Dy2TiO5.[9] In this instance, Dy3+ is 5-coordinated with oxygen, which produces an orthorhombic structure in the Dy2TiO5 phase. This phase also possesses a large neutron absorption cross section, which makes it desirable for various nuclear applications.[3] This can, however, pose difficulties when characterizing this compound through the use of neutron diffraction.[10]
Synthesis
Dysprosium titanate can be synthesized using various methods. The traditional synthesis process involve high-frequency induction melting of dysprosium oxide and titania in a cooled crucible. Sol-gel synthesis has also been utilized as a method to produce the compound in powder form. More recent developments have displayed the viability of mechanochemical processes using anatase and dysprosium oxide as reagents to produce dysprosium titanate nanopowders.[11][12]
Uses and Applications
Dysprosium titanate has become a desirable material in nuclear industry because of various properties. The compound has a large neutron absorption cross-section, low thermal expansion, high heat capacity, high radiation resistance, and a high melting point,[13][14] all of which make dysprosium titanate a favorable material to use in control rods for nuclear reactors.[2][12]
Specifically, this material is used in the control rods for industrial thermal neutron reactors such as the VVER-1000 reactor type.[15]
References
- ↑ 1.0 1.1 Dolgikh V.A., Lavat E.A. (1991). "Preparation of new oxide nitrides with the pyrochlore structure". Russ. J. Inorg. Chem. 36: 1389–1392.
- ↑ 2.0 2.1 2.2 Sherrod, Roman; O'Quinn, Eric C.; Gussev, Igor M.; Overstreet, Cale; Neuefeind, Joerg; Lang, Maik K. (2021-04-16). "Comparison of short-range order in irradiated dysprosium titanates" (in en). npj Materials Degradation 5 (1): 19. doi:10.1038/s41529-021-00165-6. ISSN 2397-2106. Bibcode: 2021npjMD...5...19S.
- ↑ 3.0 3.1 Risovany, V.D.; Varlashova, E.E.; Suslov, D.N. (2000). "Dysprosium titanate as an absorber material for control rods". Journal of Nuclear Materials 281 (1): 84–89. doi:10.1016/S0022-3115(00)00129-X. Bibcode: 2000JNuM..281...84R.
- ↑ 4.0 4.1 Gardner, Jason S. (2010). "Magnetic pyrochlore oxides". Reviews of Modern Physics 82 (1): 53–107. doi:10.1103/RevModPhys.82.53. Bibcode: 2010RvMP...82...53G. https://journals.aps.org/rmp/abstract/10.1103/RevModPhys.82.53.
- ↑ Harris, M. J. (1997). "Geometrical Frustration in the Ferromagnetic Pyrochlore Ho2Ti2O7". Physical Review Letters 79 (13): 2554–2557. doi:10.1103/PhysRevLett.79.2554. https://journals.aps.org/prl/abstract/10.1103/PhysRevLett.79.2554.
- ↑ "Magnetic Monopoles Detected In A Real Magnet For The First Time". Science Daily. 2009-09-04. https://www.sciencedaily.com/releases/2009/09/090903163725.htm.
- ↑ Morris, D. J. P.; Tennant, D. A.; Grigera, S. A.; Klemke, B.; Castelnovo, C.; Moessner, R.; Czternasty, C.; Meissner, M. et al. (2009-10-16). "Dirac Strings and Magnetic Monopoles in the Spin Ice Dy2Ti2O7". Science 326 (5951): 411–414. doi:10.1126/science.1178868. PMID 19729617. https://www.science.org/doi/10.1126/science.1178868.
- ↑ Scharffe, S.; Kolland, G.; Valldor, M.; Cho, V.; Welter, J. F.; Lorenz, T. (2015-06-01). "Heat transport of the spin-ice materials Ho2Ti2O7 and Dy2Ti2O7". Journal of Magnetism and Magnetic Materials. Selected papers from the sixth Moscow International Symposium on Magnetism (MISM-2014) 383: 83–87. doi:10.1016/j.jmmm.2014.11.015. ISSN 0304-8853. https://www.sciencedirect.com/science/article/abs/pii/S0304885314011238.
- ↑ Aughterson, Robert D.; Lumpkin, Gregory R.; Thorogood, Gordon J.; Zhang, Zhaoming; Gault, Baptiste; Cairney, Julie M. (2015-07-01). "Crystal chemistry of the orthorhombic Ln2TiO5 compounds with Ln=La, Pr, Nd, Sm, Gd, Tb and Dy". Journal of Solid State Chemistry 227: 60–67. doi:10.1016/j.jssc.2015.03.003. ISSN 0022-4596. https://www.sciencedirect.com/science/article/abs/pii/S002245961500081X.
- ↑ Shamblin, Jacob (2016). "Crystal structure and partial Ising-like magnetic ordering of orthorhombic Dy2TiO5". Physical Review B 94 (2). doi:10.1103/PhysRevB.94.024413. Bibcode: 2016PhRvB..94b4413S. https://journals.aps.org/prb/abstract/10.1103/PhysRevB.94.024413.
- ↑ Sharipzyanova, G. Kh.; Eremeeva, Zh. V.; Karlina, Y. I. (2024-03-01). "Study of the mechanical properties of dysprosium-titanate and dysprosium-hafnate nanopowders" (in en). Metallurgist 67 (11): 1971–1977. doi:10.1007/s11015-024-01695-5. ISSN 1573-8892. https://link.springer.com/article/10.1007/s11015-024-01695-5.
- ↑ 12.0 12.1 Eremeeva, Zh. V.; Panov, V. S.; Myakisheva, L. V.; Lizunov, A. V.; Nepapushev, A. A.; Sidorenko, D. A.; Vorotilo, S. (2017-11-01). "Structure and properties of mechanochemically synthesized dysprosium titanate Dy2TiO5". Journal of Nuclear Materials 495: 38–48. doi:10.1016/j.jnucmat.2017.07.058. ISSN 0022-3115. Bibcode: 2017JNuM..495...38E. https://www.sciencedirect.com/science/article/abs/pii/S0022311517300594.
- ↑ Panneerselvam, G; Venkata Krishnan, R; Antony, M. P; Nagarajan, K; Vasudevan, T; Vasudeva Rao, P. R (2004-05-01). "Thermophysical measurements on dysprosium and gadolinium titanates". Journal of Nuclear Materials 327 (2): 220–225. doi:10.1016/j.jnucmat.2004.02.009. ISSN 0022-3115. Bibcode: 2004JNuM..327..220P. https://www.sciencedirect.com/science/article/abs/pii/S0022311504000807.
- ↑ Lee, Byung-Ho; Kim, Han-Soo; Lee, Sang-Hyun; Sohn, Dong-Seong (2007-04-01). "Measurement of the thermal properties of gadolinium and dysprosium titanate". Thermochimica Acta. 6th KSTP Symposium 455 (1): 100–104. doi:10.1016/j.tca.2006.11.033. ISSN 0040-6031. Bibcode: 2007TcAc..455..100L. https://www.sciencedirect.com/science/article/abs/pii/S004060310600606X.
- ↑ Risovany, V. D.; Varlashova, E. E.; Suslov, D. N. (2000-09-02). "Dysprosium titanate as an absorber material for control rods". Journal of Nuclear Materials 281 (1): 84–89. doi:10.1016/S0022-3115(00)00129-X. ISSN 0022-3115. Bibcode: 2000JNuM..281...84R. https://www.sciencedirect.com/science/article/abs/pii/S002231150000129X.
 |
 KSF
KSF