E-64
Topic: Chemistry
 From HandWiki - Reading time: 2 min
From HandWiki - Reading time: 2 min
| |
| |
| Names | |
|---|---|
| IUPAC name
(1S,2S)-2-(((S)-1-((4-Guanidinobutyl)amino)-4-methyl-1-oxopentan-2-yl)carbamoyl)cyclopropanecarboxylic acid
| |
| Other names
E 64; Proteinase Inhibitor E 64; N-[N-(L-3-trans-carboxyirane-2-carbonyl)-L-leucyl]-agmatine; [1-[N-[(L-3-trans-carboxyoxirane-2-carbonyl)-L-leucyl]amino]-4-guanidinobutane]
| |
| Identifiers | |
3D model (JSmol)
|
|
| 6666631 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| EC Number |
|
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C15H27N5O5 | |
| Molar mass | 357.411 g·mol−1 |
| Hazards | |
| GHS pictograms | 
|
| GHS Signal word | Warning |
| H371 | |
| P260, P264, P270, P309+311, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
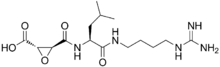
E-64 is an epoxide which can irreversibly inhibit a wide range of cysteine peptidases.
The compound was first isolated and identified from Aspergillus japonicus in 1978.[1] It has since been shown to inhibit many cysteine peptidases such as papain, cathepsin B, cathepsin L, calpain and staphopain.[2]
The low toxic effects of the inhibitor, in addition to its effective mechanism of action, makes E-64 a potential template for drugs to treat diseases where high levels of a cysteine proteases are the primary cause.
Structure and mechanism of inhibition
E-64 possesses a trans-epoxysuccinic acid group coupled to a modified dipeptide. The covalent attachment of E-64 to the active site cysteine occurs via nucleophillic attack from the thiol group of the cysteine on C2 of the epoxide. Early studies suggested that the amino-4-guanidinobutane bound in the S3' subsite and the leucyl group in the S2' subsite,[3] however published crystal structures of E-64 complexed with papain indicated that E-64 binds via the S subsites.[2]

See also
References
- ↑ "Isolation and Characterization of E–64, a New Thiol Protease Inhibitor". Agric. Biol. Chem. 42 (3): 523–528. 1978. doi:10.1080/00021369.1978.10863014.
- ↑ 2.0 2.1 "Crystal structure of a papain-E-64 complex". Biochemistry 28 (3): 1332–2. 1989. doi:10.1021/bi00429a058. PMID 2713367.
- ↑ "L-trans-Epoxysuccinyl-leucylamido(4-guanidino)butane (E-64) and its analogues as inhibitors of cysteine proteinases including cathepsins B, H and L.". Biochem. J. 201 (1): 189–98. 1982. doi:10.1042/bj2010189. PMID 7044372.
 |
 KSF
KSF