Extraction
Topic: Chemistry
 From HandWiki - Reading time: 6 min
From HandWiki - Reading time: 6 min


Extraction in chemistry is a separation process consisting of the separation of a substance from a matrix. The distribution of a solute between two phases is an equilibrium condition described by partition theory. This is based on exactly how the analyte moves from the initial solvent into the extracting solvent. The term washing may also be used to refer to an extraction in which impurities are extracted from the solvent containing the desired compound.
Types of extraction
- Liquid–liquid extraction
- Solid-liquid extraction
- Solid-phase extraction
- Maceration
- Ultrasound-assisted extraction
- Microwave-assisted extraction
- Heat reflux extraction
- Instant controlled pressure drop extraction (Détente instantanée contrôlée)
- Perstraction
Laboratory applications and examples
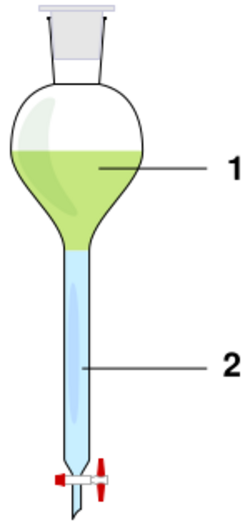
Liquid-liquid extractions in the laboratory usually make use of a separatory funnel, where two immiscible phases are combined to separate a solute from one phase into the other, according to the relative solubility in each of the phases. Typically, this will be to extract organic compounds out of an aqueous phase and into an organic phase, but may also include extracting water-soluble impurities from an organic phase into an aqueous phase.[1][2]
Common extractants may be arranged in increasing order of polarity according to the Hildebrand solubility parameter:
ethyl acetate < acetone < ethanol < methanol < acetone:water (7:3) < ethanol:water (8:2) < methanol:water (8:2) < water
Solid-liquid extractions at laboratory scales can use Soxhlet extractors. A solid sample containing the desired compound along with impurities is placed in the thimble. An extracting solvent is chosen in which the impurities are insoluble and the desired compound has at least limited solubility. The solvent is refluxed and condensed solvent falls into the thimble and dissolves the desired compound which then passes back through the filter into the flask. After extraction is complete the solvent can be removed and the desired product collected.
Everyday applications and examples
Boiling tea leaves in water extracts the tannins, theobromine, and caffeine out of the leaves and into the water, as an example of a solid-liquid extraction.
Decaffeination of tea and coffee is also an example of an extraction, where the caffeine molecules are removed from the tea leaves or coffee beans, often utilising supercritical fluid extraction with CO2 or standard solid-liquid extraction techniques.[3]
See also
- Sample preparation (analytical chemistry)
- Solvent
- Solvent impregnated resins
- Thin Layer Extraction
- Leaching (Chemistry)
References
- ↑ "4: Extraction" (in en). 2017-10-05. https://chem.libretexts.org/Bookshelves/Organic_Chemistry/Book%3A_Organic_Chemistry_Lab_Techniques_(Nichols)/4%3A_Extraction.
- ↑ Zubrick, James W. (2014). The organic chem lab survival manual : a student's guide to techniques (Ninth ed.). Hoboken: John Wiley & Sons. pp. 127–144. ISBN 9781118083390. OCLC 798220947.
- ↑ Ramalakshmi, K.; Raghavan, B. (1999). "Caffeine in Coffee: Its Removal. Why and How?" (in en). Critical Reviews in Food Science and Nutrition 39 (5): 441–456. doi:10.1080/10408699991279231. ISSN 1040-8398. PMID 10516914.
Further reading
- Fundamentals of Analytical Chemistry (8th ed.).
- Gunt Hamburg, 2014, Thermal Process Engineering: Liquid-liquid extraction and solid-liquid extraction, see [1], accessed 12 May 2014.
- Colin Poole & Michael Cooke, 2000, Extraction, in Encyclopedia of Separation Science, 10 Vols., ISBN 9780122267703, accessed 12 May 2014.
- Stevens, G.W.; Lo, Teh C.; Baird, Malcolm H. I. (2007). "Extraction, Liquid-Liquid" (in en). Kirk-Othmer Encyclopedia of Chemical Technology (1 ed.). Wiley. doi:10.1002/0471238961.120917211215.a01.pub2. ISBN 978-0-471-48494-3. https://onlinelibrary.wiley.com/doi/10.1002/0471238961.120917211215.a01.pub2. Retrieved 12 May 2014.
- Voeste, T.; Weber, K.; Hiskey, B.; Brunner, G. (2006). "Liquid–Solid Extraction" (in en). Ullmann's Encyclopedia of Industrial Chemistry (1 ed.). Wiley. doi:10.1002/14356007.b03_07.pub2. doi:10.1002/14356007.b03_07.pub2. ISBN 978-3-527-30385-4. https://onlinelibrary.wiley.com/doi/10.1002/14356007.b03_07.pub2. Retrieved 12 May 2014.
- R. J. Wakeman, 2000, "Extraction, Liquid-Solid", in Kirk-Othmer Encyclopedia of Chemical Technology, doi:10.1002/0471238961.1209172123011105.a01, accessed 12 May 2014.
- M.J.M. Wells, 2000, "Essential guides to method development in solid-phase extraction," in Encyclopedia of Separation Science, Vol. 10 (I.D. Wilson, E.R. Adlard, M. Cooke, and C.F. Poole, eds.), London:Academic Press, London, 2000, pp. 4636–4643. ISBN 978-0122267703
External links
 KSF
KSF