Fucose
Topic: Chemistry
 From HandWiki - Reading time: 5 min
From HandWiki - Reading time: 5 min
| |
| |
| Names | |
|---|---|
| IUPAC name
6-Deoxy-L-galactopyranose
| |
| Systematic IUPAC name
(2S,3R,4R,5S)-6-Methyltetrahydro- | |
| Other names
6-Deoxy-l-galactose
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C6H12O5 | |
| Molar mass | 164.16 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Fucose is a hexose deoxy sugar with the chemical formula C6H12O5. It is found on N-linked glycans on the mammalian, insect and plant cell surface. Fucose is the fundamental sub-unit of the seaweed polysaccharide fucoidan.[1] The α(1→3) linked core of fucoidan is a suspected carbohydrate antigen for IgE-mediated allergy.[2]
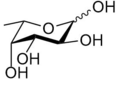
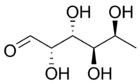
Two structural features distinguish fucose from other six-carbon sugars present in mammals: the lack of a hydroxyl group on the carbon at the 6-position (C-6) (thereby making it a deoxy sugar) and the L-configuration. It is equivalent to 6-deoxy-l-galactose.
In the fucose-containing glycan structures, fucosylated glycans, fucose can exist as a terminal modification or serve as an attachment point for adding other sugars.[3] In human N-linked glycans, fucose is most commonly linked α-1,6 to the reducing terminal β-N-acetylglucosamine. However, fucose at the non-reducing termini linked α-1,2 to galactose forms the H antigen, the substructure of the A and B blood group antigens.
Fucose is released from fucose-containing polymers by an enzyme called α-fucosidase found in lysosomes.
l-Fucose has several potential applications in cosmetics, pharmaceuticals, and dietary supplements[4][5]
Fucosylation of antibodies has been established to reduce binding to the Fc receptor of Natural Killer cells and thereby reduce antigen-dependent cellular cytotoxicity. Therefore, afucosylated monoclonal antibodies have been designed to recruit the immune system to cancers cells have been manufactured in cell lines deficient in the enzyme for core fucosylation (FUT8), thereby enhancing the in vivo cell killing.[6][7]
See also
- Digitalose, the methyl ether of D-fucose
- Fucitol
- Verotoxin-producing Escherichia coli
References
- ↑ Garcia-Vaquero, M.; Rajauria, G.; O'Doherty, J.V.; Sweeney, T. (2017-09-01). "Polysaccharides from macroalgae: Recent advances, innovative technologies and challenges in extraction and purification" (in en). Food Research International 99 (Pt 3): 1011–1020. doi:10.1016/j.foodres.2016.11.016. ISSN 0963-9969. PMID 28865611.
- ↑
 Daniel J. Becker; John B. Lowe (July 2003). "Fucose: biosynthesis and biological function in mammals". Glycobiology 13 (7): 41R–53R. doi:10.1093/glycob/cwg054. PMID 12651883.
Daniel J. Becker; John B. Lowe (July 2003). "Fucose: biosynthesis and biological function in mammals". Glycobiology 13 (7): 41R–53R. doi:10.1093/glycob/cwg054. PMID 12651883.
- ↑
 Daniel J. Moloney; Robert S. Haltiwanger (July 1999). "The O-linked fucose glycosylation pathway: identification and characterization of a uridine diphosphoglucose: fucose-[beta]1,3-glucosyltransferase activity from Chinese hamster ovary cells". Glycobiology 9 (7): 679–687. doi:10.1093/glycob/9.7.679. PMID 10362837.
Daniel J. Moloney; Robert S. Haltiwanger (July 1999). "The O-linked fucose glycosylation pathway: identification and characterization of a uridine diphosphoglucose: fucose-[beta]1,3-glucosyltransferase activity from Chinese hamster ovary cells". Glycobiology 9 (7): 679–687. doi:10.1093/glycob/9.7.679. PMID 10362837.
- ↑ Roca, C (2015). "Exopolysaccharides enriched in rare sugars: bacterial sources, production, and applications". Front Microbiol 6: 288. doi:10.3389/fmicb.2015.00288. PMID 25914689.
- ↑ Vanhooren, PT (1999). "L-fucose: occurrence, physiological role, chemical, enzymatic and microbial synthesis". J. Chem. Technol. Biotechnol. 74 (6): 479–497. doi:10.1002/(SICI)1097-4660(199906)74:6<479::AID-JCTB76>3.0.CO;2-E.
- ↑ Dalziel, Martin; Crispin, Max; Scanlan, Christopher N.; Zitzmann, Nicole; Dwek, Raymond A. (2014-01-03). "Emerging Principles for the Therapeutic Exploitation of Glycosylation" (in en). Science 343 (6166): 1235681. doi:10.1126/science.1235681. ISSN 0036-8075. PMID 24385630.
- ↑ Yu, X; Marshall, MJE; Cragg, MS; Crispin, M (June 2017). "Improving Antibody-Based Cancer Therapeutics Through Glycan Engineering.". BioDrugs 31 (3): 151–166. doi:10.1007/s40259-017-0223-8. PMID 28466278. https://eprints.soton.ac.uk/410615/1/Resubmission_Yu_Manuscript_and_table.pdf.
 |
 KSF
KSF