Glucocorticoid
Topic: Chemistry
 From HandWiki - Reading time: 22 min
From HandWiki - Reading time: 22 min
| Glucocorticoid | |
|---|---|
| Drug class | |
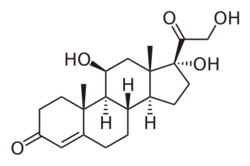 Chemical structure of cortisol (hydrocortisone), an endogenous glucocorticoid as well as a medication. | |
| Class identifiers | |
| Synonyms | Corticosteroid; Glucocorticosteroid |
| Use | Adrenal insufficiency; allergic, inflammatory, and autoimmune disorders; asthma; organ transplant |
| ATC code | H02AB |
| Biological target | Glucocorticoid receptor |
| Chemical class | Steroids |
Glucocorticoids (or, less commonly, glucocorticosteroids) are a class of corticosteroids, which are a class of steroid hormones. Glucocorticoids are corticosteroids that bind to the glucocorticoid receptor[1] that is present in almost every vertebrate animal cell. The name "glucocorticoid" is a portmanteau (glucose + cortex + steroid) and is composed from its role in regulation of glucose metabolism, synthesis in the adrenal cortex, and its steroidal structure (see structure below).
Glucocorticoids are part of the feedback mechanism in the immune system, which reduces certain aspects of immune function, such as inflammation. They are therefore used in medicine to treat diseases caused by an overactive immune system, such as allergies, asthma, autoimmune diseases, and sepsis. Glucocorticoids have many diverse effects such as pleiotropy, including potentially harmful side effects.[2] They also interfere with some of the abnormal mechanisms in cancer cells, so they are used in high doses to treat cancer. This includes inhibitory effects on lymphocyte proliferation, as in the treatment of lymphomas and leukemias, and the mitigation of side effects of anticancer drugs.
Glucocorticoids affect cells by binding to the glucocorticoid receptor. The activated glucocorticoid receptor-glucocorticoid complex up-regulates the expression of anti-inflammatory proteins in the nucleus (a process known as transactivation) and represses the expression of proinflammatory proteins in the cytosol by preventing the translocation of other transcription factors from the cytosol into the nucleus (transrepression).[2]
Glucocorticoids are distinguished from mineralocorticoids and sex steroids by their specific receptors, target cells, and effects. In technical terms, "corticosteroid" refers to both glucocorticoids and mineralocorticoids (as both are mimics of hormones produced by the adrenal cortex), but is often used as a synonym for "glucocorticoid". Glucocorticoids are chiefly produced in the zona fasciculata of the adrenal cortex, whereas mineralocorticoids are synthesized in the zona glomerulosa.
Cortisol (or hydrocortisone) is the most important human glucocorticoid. It is essential for life, and it regulates or supports a variety of important cardiovascular, metabolic, immunologic, and homeostatic functions. Various synthetic glucocorticoids are available; these are widely utilized in general medical practice and numerous specialties, either as replacement therapy in glucocorticoid deficiency or to suppress the body's immune system.
Effects
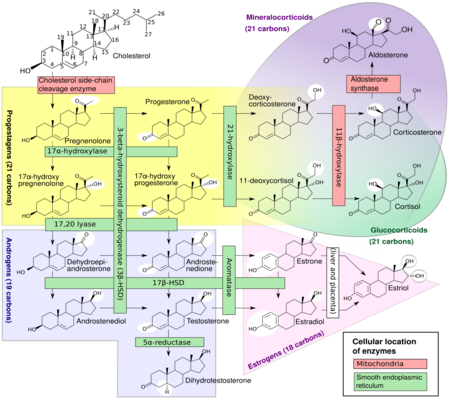
Glucocorticoid effects may be broadly classified into two major categories: immunological and metabolic. In addition, glucocorticoids play important roles in fetal development and body fluid homeostasis.
Immune
Glucocorticoids function via interaction with the glucocorticoid receptor (see details below):
- Upregulate the expression of anti-inflammatory proteins.
- Downregulate the expression of proinflammatory proteins.
Glucocorticoids are also shown to play a role in the development and homeostasis of T lymphocytes. This has been shown in transgenic mice with either increased or decreased sensitivity of T cell lineage to glucocorticoids.[4]
Metabolic
The name "glucocorticoid" derives from early observations that these hormones were involved in glucose metabolism. In the fasted state, cortisol stimulates several processes that collectively serve to increase and maintain normal concentrations of glucose in the blood.
Metabolic effects:
- Stimulation of gluconeogenesis, in particular, in the liver: This pathway results in the synthesis of glucose from non-hexose substrates, such as amino acids and glycerol from triglyceride breakdown, and is particularly important in carnivores and certain herbivores. Enhancing the expression of enzymes involved in gluconeogenesis is probably the best-known metabolic function of glucocorticoids.
- Mobilization of amino acids from extrahepatic tissues: These serve as substrates for gluconeogenesis.
- Inhibition of glucose uptake in muscle and adipose tissue: A mechanism to conserve glucose
- Stimulation of fat breakdown in adipose tissue: The fatty acids released by lipolysis are used for production of energy in tissues like muscle, and the released glycerol provide another substrate for gluconeogenesis.
- Increase in sodium retention and potassium excretion leads to hypernatremia and hypokalemia[5]
- Increase in hemoglobin concentration, likely due to hindrance of the ingestion of red blood cell by macrophage or other phagocyte.[1]
- Increased urinary uric acid[6]
- Increased urinary calcium and hypocalcemia[7]
- Alkalosis[8]
- Leukocytosis[9]
Excessive glucocorticoid levels resulting from administration as a drug or hyperadrenocorticism have effects on many systems. Some examples include inhibition of bone formation, suppression of calcium absorption (both of which can lead to osteoporosis), delayed wound healing, muscle weakness, and increased risk of infection. These observations suggest a multitude of less-dramatic physiologic roles for glucocorticoids.[4]
Developmental
Glucocorticoids have multiple effects on fetal development. An important example is their role in promoting maturation of the lung and production of the surfactant necessary for extrauterine lung function. Mice with homozygous disruptions in the corticotropin-releasing hormone gene (see below) die at birth due to pulmonary immaturity. In addition, glucocorticoids are necessary for normal brain development, by initiating terminal maturation, remodeling axons and dendrites, and affecting cell survival[8] and may also play a role in hippocampal development. Glucocorticoids stimulate the maturation of the Na+/K+/ATPase, nutrient transporters, and digestion enzymes, promoting the development of a functioning gastro-intestinal system. Glucocorticoids also support the development of the neonate's renal system by increasing glomerular filtration.
Arousal and cognition
Glucocorticoids act on the hippocampus, amygdala, and frontal lobes. Along with adrenaline, these enhance the formation of flashbulb memories of events associated with strong emotions, both positive and negative.[9] This has been confirmed in studies, whereby blockade of either glucocorticoids or noradrenaline activity impaired the recall of emotionally relevant information. Additional sources have shown subjects whose fear learning was accompanied by high cortisol levels had better consolidation of this memory (this effect was more important in men).[better source needed] The effect that glucocorticoids have on memory may be due to damage specifically to the CA1 area of the hippocampal formation.
In multiple animal studies, prolonged stress (causing prolonged increases in glucocorticoid levels) have shown destruction of the neurons in the hippocampus area of the brain, which has been connected to lower memory performance.[5][10][6]
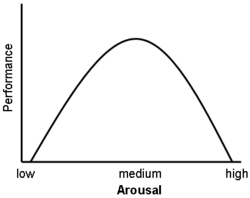
Glucocorticoids have also been shown to have a significant impact on vigilance (attention deficit disorder) and cognition (memory). This appears to follow the Yerkes-Dodson curve, as studies have shown circulating levels of glucocorticoids vs. memory performance follow an upside-down U pattern, much like the Yerkes-Dodson curve. For example, long-term potentiation (LTP; the process of forming long-term memories) is optimal when glucocorticoid levels are mildly elevated, whereas significant decreases of LTP are observed after adrenalectomy (low-glucocorticoid state) or after exogenous glucocorticoid administration (high-glucocorticoid state). Elevated levels of glucocorticoids enhance memory for emotionally arousing events, but lead more often than not to poor memory for material unrelated to the source of stress/emotional arousal.[11] In contrast to the dose-dependent enhancing effects of glucocorticoids on memory consolidation, these stress hormones have been shown to inhibit the retrieval of already stored information.[7] Long-term exposure to glucocorticoid medications, such as asthma and anti-inflammatory medication, has been shown to create deficits in memory and attention both during and, to a lesser extent, after treatment,[12][13] a condition known as "steroid dementia".[14]
Body fluid homeostasis
Glucocorticoids could act centrally, as well as peripherally, to assist in the normalization of extracellular fluid volume by regulating body's action to atrial natriuretic peptide (ANP). Centrally, glucocorticoids could inhibit dehydration induce water intake;[15] peripherally, glucocorticoids could induce a potent diuresis.[16]
Mechanism of action
Transactivation
Glucocorticoids bind to the cytosolic glucocorticoid receptor, a type of nuclear receptor that is activated by ligand binding. After a hormone binds to the corresponding receptor, the newly formed complex translocates itself into the cell nucleus, where it binds to glucocorticoid response elements in the promoter region of the target genes resulting in the regulation of gene expression. This process is commonly referred to as transcriptional activation, or transactivation.[17][18]
The proteins encoded by these up-regulated genes have a wide range of effects, including, for example:[18]
- Anti-inflammatory – lipocortin I, p11/calpactin binding protein, secretory leukocyte protease inhibitor 1 (SLPI), and Mitogen-activated protein kinase phosphatase (MAPK phosphatase)
- Increased gluconeogenesis – glucose 6-phosphatase and tyrosine aminotransferase
Transrepression
The opposite mechanism is called transcriptional repression, or transrepression. The classical understanding of this mechanism is that activated glucocorticoid receptor binds to DNA in the same site where another transcription factor would bind, which prevents the transcription of genes that are transcribed via the activity of that factor.[17][18] While this does occur, the results are not consistent for all cell types and conditions; there is no generally accepted, general mechanism for transrepression.[18]
New mechanisms are being discovered where transcription is repressed, but the activated glucocorticoid receptor is not interacting with DNA, but rather with another transcription factor directly, thus interfering with it, or with other proteins that interfere with the function of other transcription factors. This latter mechanism appears to be the most likely way that activated glucocorticoid receptor interferes with NF-κB - namely by recruiting histone deacetylase, which deacetylate the DNA in the promoter region leading to closing of the chromatin structure where NF-κB needs to bind.[17][18]
Nongenomic effects
Activated glucocorticoid receptor has effects that have been experimentally shown to be independent of any effects on transcription and can only be due to direct binding of activated glucocorticoid receptor with other proteins or with mRNA.[17][18]
For example, Src kinase which binds to inactive glucocorticoid receptor, is released when a glucocorticoid binds to glucocorticoid receptor, and phosphorylates a protein that in turn displaces an adaptor protein from a receptor important in inflammation, epidermal growth factor, reducing its activity, which in turn results in reduced creation of arachidonic acid – a key proinflammatory molecule. This is one mechanism by which glucocorticoids have an anti-inflammatory effect.[17]
Pharmacology
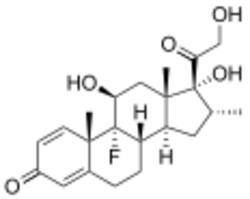
A variety of synthetic glucocorticoids, some far more potent than cortisol, have been created for therapeutic use. They differ in both pharmacokinetics (absorption factor, half-life, volume of distribution, clearance) and pharmacodynamics (for example the capacity of mineralocorticoid activity: retention of sodium (Na+) and water; renal physiology). Because they permeate the intestines easily, they are administered primarily per os (by mouth), but also by other methods, such as topically on skin. More than 90% of them bind different plasma proteins, though with a different binding specificity. Endogenous glucocorticoids and some synthetic corticoids have high affinity to the protein transcortin (also called corticosteroid-binding globulin), whereas all of them bind albumin. In the liver, they quickly metabolize by conjugation with a sulfate or glucuronic acid, and are secreted in the urine.
Glucocorticoid potency, duration of effect, and the overlapping mineralocorticoid potency vary. Cortisol is the standard of comparison for glucocorticoid potency. Hydrocortisone is the name used for pharmaceutical preparations of cortisol.
The data below refer to oral administration. Oral potency may be less than parenteral potency because significant amounts (up to 50% in some cases) may not reach the circulation. Fludrocortisone acetate and deoxycorticosterone acetate are, by definition, mineralocorticoids rather than glucocorticoids, but they do have minor glucocorticoid potency and are included in this table to provide perspective on mineralocorticoid potency.
| Name | Glucocorticoid potency | Mineralocorticoid potency | Terminal half-life (hours) |
|---|---|---|---|
| Cortisol (hydrocortisone) | 1 | 1 | 8 |
| Cortisone | 0.8 | 0.8 | 8 |
| Prednisone | 3.5–5 | 0.8 | 16–36 |
| Prednisolone | 4 | 0.8 | 16–36 |
| Methylprednisolone | 5–7.5 | 0.5 | 18–40 |
| Dexamethasone | 25–80 | 0 | 36–54 |
| Betamethasone | 25–30 | 0 | 36–54 |
| Triamcinolone | 5 | 0 | 12–36 |
| Deflazacort | 6.5 | – | 1.3 |
| Fludrocortisone acetate | 15 | 200 | 24 |
| Deoxycorticosterone acetate | 0 | 20 | – |
| Aldosterone | 0.3 | 200–1000 | – |
| Beclometasone | 8 sprays 4 times every day equivalent to orally 14 mg prednisone once a day | – | – |
Therapeutic use
Glucocorticoids may be used in low doses in adrenal insufficiency. In much higher doses, oral or inhaled glucocorticoids are used to suppress various allergic, inflammatory, and autoimmune disorders. Inhaled glucocorticoids are the second-line treatment for asthma. They are also administered as post-transplantory immunosuppressants to prevent the acute transplant rejection and the graft-versus-host disease. Nevertheless, they do not prevent an infection and also inhibit later reparative processes. Newly emerging evidence showed that glucocorticoids could be used in the treatment of heart failure to increase the renal responsiveness to diuretics and natriuretic peptides. Glucocorticoids are historically used for pain relief in inflammatory conditions.[23][24][25] However, corticosteroids show limited efficacy in pain relief and potential adverse events for their use in tendinopathies.[26]
Replacement
Any glucocorticoid can be given in a dose that provides approximately the same glucocorticoid effects as normal cortisol production; this is referred to as physiologic, replacement, or maintenance dosing. This is approximately 6–12 mg/m2/day of hydrocortisone (m2 refers to body surface area (BSA), and is a measure of body size; an average man's BSA is 1.9 m2).
Therapeutic immunosuppression
Glucocorticoids cause immunosuppression, and the therapeutic component of this effect is mainly the decreases in the function and numbers of lymphocytes, including both B cells and T cells.
The major mechanism for this immunosuppression is through inhibition of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB). NF-κB is a critical transcription factor involved in the synthesis of many mediators (i.e., cytokines) and proteins (i.e., adhesion proteins) that promote the immune response. Inhibition of this transcription factor, therefore, blunts the capacity of the immune system to mount a response.[2]
Glucocorticoids suppress cell-mediated immunity by inhibiting genes that code for the cytokines IL-1, IL-2, IL-3, IL-4, IL-5, IL-6, IL-8 and IFN-γ, the most important of which is IL-2. Smaller cytokine production reduces the T cell proliferation.[27]
Glucocorticoids, however, not only reduce T cell proliferation, but also lead to another well known effect - glucocorticoid-induced apoptosis. The effect is more prominent in immature T cells still inside in the thymus, but peripheral T cells are also affected. The exact mechanism regulating this glucocorticoid sensitivity lies in the Bcl-2 gene.[28]
Glucocorticoids also suppress the humoral immunity, thereby causing a humoral immune deficiency. Glucocorticoids cause B cells to express smaller amounts of IL-2 and of IL-2 receptors. This diminishes both B cell clone expansion and antibody synthesis. The diminished amounts of IL-2 also cause fewer T lymphocyte cells to be activated.
The effect of glucocorticoids on Fc receptor expression in immune cells is complicated. Dexamethasone decreases IFN-gamma stimulated Fc gamma RI expression in neutrophils while conversely causing an increase in monocytes.[29] Glucocorticoids may also decrease the expression of Fc receptors in macrophages,[30] but the evidence supporting this regulation in earlier studies has been questioned.[31] The effect of Fc receptor expression in macrophages is important since it is necessary for the phagocytosis of opsonised cells. This is because Fc receptors bind antibodies attached to cells targeted for destruction by macrophages.
Anti-inflammatory
Glucocorticoids are potent anti-inflammatories, regardless of the inflammation's cause; their primary anti-inflammatory mechanism is lipocortin-1 (annexin-1) synthesis. Lipocortin-1 both suppresses phospholipase A2, thereby blocking eicosanoid production, and inhibits various leukocyte inflammatory events (epithelial adhesion, emigration, chemotaxis, phagocytosis, respiratory burst, etc.). In other words, glucocorticoids not only suppress immune response, but also inhibit the two main products of inflammation, prostaglandins and leukotrienes. They inhibit prostaglandin synthesis at the level of phospholipase A2 as well as at the level of cyclooxygenase/PGE isomerase (COX-1 and COX-2),[32] the latter effect being much like that of NSAIDs, thus potentiating the anti-inflammatory effect.
In addition, glucocorticoids also suppress cyclooxygenase expression.[33]
Glucocorticoids marketed as anti-inflammatories are often topical formulations, such as nasal sprays for rhinitis or inhalers for asthma. These preparations have the advantage of only affecting the targeted area, thereby reducing side effects or potential interactions. In this case, the main compounds used are beclometasone, budesonide, fluticasone, mometasone and ciclesonide. In rhinitis, sprays are used. For asthma, glucocorticoids are administered as inhalants with a metered-dose or dry powder inhaler.[34] In rare cases, symptoms of radiation induced thyroiditis has been treated with oral glucocorticoids.[35]
Hyperaldosteronism
Glucocorticoids can be used in the management of familial hyperaldosteronism type 1. They are not effective, however, for use in the type 2 condition.
Heart failure
Glucocorticoids could be used in the treatment of decompensated heart failure to potentiate renal responsiveness to diuretics, especially in heart failure patients with refractory diuretic resistance with large doses of loop diuretics.[36][37][38][39][40][41][42]
Resistance

Resistance to the therapeutic uses of glucocorticoids can present difficulty; for instance, 25% of cases of severe asthma may be unresponsive to steroids. This may be the result of genetic predisposition, ongoing exposure to the cause of the inflammation (such as allergens), immunological phenomena that bypass glucocorticoids, pharmacokinetic disturbances (incomplete absorption or accelerated excretion or metabolism) and viral and/or bacterial respiratory infections.[27][43]
Side effects
Glucocorticoid drugs currently being used act nonselectively, so in the long run they may impair many healthy anabolic processes. To prevent this, much research has been focused recently on the elaboration of selectively acting glucocorticoid drugs. Side effects include:
- Immunodeficiency (see section below)
- Hyperglycemia due to increased gluconeogenesis, insulin resistance, and impaired glucose tolerance ("steroid diabetes"); caution in those with diabetes mellitus
- Increased skin fragility, easy bruising
- Negative calcium balance due to reduced intestinal calcium absorption[44]
- Steroid-induced osteoporosis: reduced bone density (osteoporosis, osteonecrosis, higher fracture risk, slower fracture repair)
- Weight gain due to increased visceral and truncal fat deposition (central obesity) and appetite stimulation; see corticosteroid-induced lipodystrophy
- Hypercortisolemia with prolonged or excessive use (also known as, exogenous Cushing's syndrome)
- Impaired memory and attention deficits[45] See steroid dementia syndrome.
- Adrenal insufficiency (if used for long time and stopped suddenly without a taper)
- Muscle and tendon breakdown (proteolysis), weakness, reduced muscle mass and repair[46][26]
- Expansion of malar fat pads and dilation of small blood vessels in skin
- Lipomatosis within the epidural space[47]
- Excitatory effect on central nervous system (euphoria, psychosis)
- Anovulation, irregularity of menstrual periods
- Growth failure, delayed puberty
- Increased plasma amino acids, increased urea formation, negative nitrogen balance
- Glaucoma due to increased ocular pressure
- Cataracts
- Topical steroid withdrawal
In high doses, hydrocortisone (cortisol) and those glucocorticoids with appreciable mineralocorticoid potency can exert a mineralocorticoid effect as well, although in physiologic doses this is prevented by rapid degradation of cortisol by 11β-hydroxysteroid dehydrogenase isoenzyme 2 (11β-HSD2) in mineralocorticoid target tissues. Mineralocorticoid effects can include salt and water retention, extracellular fluid volume expansion, hypertension, potassium depletion, and metabolic alkalosis.
Immunodeficiency
Glucocorticoids cause immunosuppression, decreasing the function and/or numbers of neutrophils, lymphocytes (including both B cells and T cells), monocytes, macrophages, and the anatomical barrier function of the skin.[48] This suppression, if large enough, can cause manifestations of immunodeficiency, including T cell deficiency, humoral immune deficiency and neutropenia.
| Bacteria |
|
|---|---|
| Fungi | |
| Viruses |
|
| Other |
|
Withdrawal
In addition to the effects listed above, use of high-dose glucocorticoids for only a few days begins to produce suppression of the patient's adrenal glands suppressing hypothalamic corticotropin-releasing hormone (CRH) leading to suppressed production of adrenocorticotropic hormone (ACTH) by the anterior pituitary.[19] With prolonged suppression, the adrenal glands atrophy (physically shrink), and can take months to recover full function after discontinuation of the exogenous glucocorticoid.
During this recovery time, the patient is vulnerable to adrenal insufficiency during times of stress, such as illness. While suppressive dose and time for adrenal recovery vary widely, clinical guidelines have been devised to estimate potential adrenal suppression and recovery, to reduce risk to the patient. The following is one example:
- If patients have been receiving daily high doses for five days or less, they can be abruptly stopped (or reduced to physiologic replacement if patients are adrenal-deficient). Full adrenal recovery can be assumed to occur by a week afterward.
- If high doses were used for six to 10 days, reduce to replacement dose immediately and taper over four more days. Adrenal recovery can be assumed to occur within two to four weeks of completion of steroids.
- If high doses were used for 11–30 days, cut immediately to twice replacement, and then by 25% every four days. Stop entirely when dose is less than half of replacement. Full adrenal recovery should occur within one to three months of completion of withdrawal.
- If high doses were used more than 30 days, cut dose immediately to twice replacement, and reduce by 25% each week until replacement is reached. Then change to oral hydrocortisone or cortisone as a single morning dose, and gradually decrease by 2.5 mg each week. When the morning dose is less than replacement, the return of normal basal adrenal function may be documented by checking 0800 cortisol levels prior to the morning dose; stop drugs when 0800 cortisol is 10 μg/dl. Predicting the time to full adrenal recovery after prolonged suppressive exogenous steroids is difficult; some people may take nearly a year.
- Flare-up of the underlying condition for which steroids are given may require a more gradual taper than outlined above.
See also
- List of corticosteroids
- List of corticosteroid cyclic ketals
- List of corticosteroid esters
- Aminoglutethimide blocks glucocorticoid secretion
- GITR (glucocorticoid-induced TNF receptor)
- Glucocorticoid receptor
- Immunosuppressive drug
- Membrane glucocorticoid receptor
- Metyrapone blocks glucocorticoid secretion
- Selective glucocorticoid receptor agonist
- Topical glucocorticoids
- Topical steroid
- Steroid atrophy
- Topical steroid withdrawal
- Non-steroidal anti-inflammatory drug (NSAID)
References
- ↑ 1.0 1.1 Glucocorticoids: effects, action mechanisms, and therapeutic uses. Hauppauge, N.Y.: Nova Science. 2011. ISBN 978-1617287589.[page needed]
- ↑ 2.0 2.1 2.2 "Antiinflammatory action of glucocorticoids–new mechanisms for old drugs". The New England Journal of Medicine 353 (16): 1711–1723. Oct 2005. doi:10.1056/NEJMra050541. PMID 16236742.
- ↑ Häggström, Mikael; Richfield, David (2014). "Diagram of the pathways of human steroidogenesis". WikiJournal of Medicine 1 (1). doi:10.15347/wjm/2014.005. ISSN 2002-4436.
- ↑ 4.0 4.1 "Effects of altered glucocorticoid sensitivity in the T cell lineage on thymocyte and T cell homeostasis". FASEB Journal 16 (7): 727–729. May 2002. doi:10.1096/fj.01-0891fje. PMID 11923224.
- ↑ 5.0 5.1 Physiology of Behavior (11th ed.). New York: Allyn & Bacon. 2010. p. 605. ISBN 978-0-205-23939-9.
- ↑ 6.0 6.1 "Glucocorticoids, stress and exacerbation of excitotoxic neuron death". Seminars in Neuroscience 6 (5): 323–331. October 1994. doi:10.1006/smns.1994.1041.
- ↑ 7.0 7.1 "Stress and glucocorticoids impair retrieval of long-term spatial memory". Nature 394 (6695): 787–790. Aug 1998. doi:10.1038/29542. PMID 9723618. Bibcode: 1998Natur.394..787D.
- ↑ 8.0 8.1 "Effects of stress throughout the lifespan on the brain, behaviour and cognition". Nature Reviews. Neuroscience 10 (6): 434–445. Jun 2009. doi:10.1038/nrn2639. PMID 19401723.
- ↑ 9.0 9.1 "Mechanisms of emotional arousal and lasting declarative memory". Trends in Neurosciences 21 (7): 294–299. Jul 1998. doi:10.1016/s0166-2236(97)01214-9. PMID 9683321.
- ↑ "Corticosteroids and cognition". Journal of Psychiatric Research 35 (3): 127–145. 2001. doi:10.1016/S0022-3956(01)00018-8. PMID 11461709.
- ↑ "The effects of stress and stress hormones on human cognition: Implications for the field of brain and cognition". Brain and Cognition 65 (3): 209–237. Dec 2007. doi:10.1016/j.bandc.2007.02.007. PMID 17466428.
- ↑ "The 'steroid dementia syndrome': a possible model of human glucocorticoid neurotoxicity". Neurocase 13 (3): 189–200. Jun 2007. doi:10.1080/13554790701475468. PMID 17786779.
- ↑ "Steroid dementia: an overlooked diagnosis?". Neurology 66 (1): 155; author reply 155. Jan 2006. doi:10.1212/01.wnl.0000203713.04232.82. PMID 16401879.
- ↑ "Reversible steroid dementia in patients without steroid psychosis". The American Journal of Psychiatry 141 (3): 369–372. Mar 1984. doi:10.1176/ajp.141.3.369. PMID 6703100. http://ajp.psychiatryonline.org/article.aspx?articleid=161461.
- ↑ "Inhibition of dehydration-induced water intake by glucocorticoids is associated with activation of hypothalamic natriuretic peptide receptor-A in rat". PLOS ONE 5 (12): e15607. 2010. doi:10.1371/journal.pone.0015607. PMID 21187974. Bibcode: 2010PLoSO...515607L.
- ↑ "Glucocorticoids improve renal responsiveness to atrial natriuretic peptide by up-regulating natriuretic peptide receptor-A expression in the renal inner medullary collecting duct in decompensated heart failure". The Journal of Pharmacology and Experimental Therapeutics 339 (1): 203–209. Oct 2011. doi:10.1124/jpet.111.184796. PMID 21737535.
- ↑ 17.0 17.1 17.2 17.3 17.4 "Mechanisms generating diversity in glucocorticoid receptor signaling". Annals of the New York Academy of Sciences 1179 (1): 167–178. Oct 2009. doi:10.1111/j.1749-6632.2009.04986.x. PMID 19906239. Bibcode: 2009NYASA1179..167R. https://zenodo.org/record/1230766.
- ↑ 18.0 18.1 18.2 18.3 18.4 18.5 "Separating transrepression and transactivation: a distressing divorce for the glucocorticoid receptor?". Molecular Pharmacology 72 (4): 799–809. Oct 2007. doi:10.1124/mol.107.038794. PMID 17622575.
- ↑ 19.0 19.1 "Glucocorticoid Therapy and Adrenal Suppression". Endotext. MDText.com. 2018. https://www.ncbi.nlm.nih.gov/books/NBK279156/.
- ↑ Liapi, C; Chrousos, GP (1992). "Glucocorticoids". in Yaffe, SJ; Aranda, JV. Pediatric Pharmacology: Therapeutic Principles in Practice (2nd ed.). Philadelphia: Saunders. pp. 466–475. ISBN 978-0721629711.
- ↑ "Disease management of atopic dermatitis: a practice parameter. Joint Task Force on Practice Parameters, representing the American Academy of Allergy, Asthma and Immunology, the American College of Allergy, Asthma and Immunology, and the Joint Council of Allergy, Asthma and Immunology. Work Group on Atopic Dermatitis". Annals of Allergy, Asthma & Immunology 79 (3): 197–211. Sep 1997. doi:10.1016/S1081-1206(10)63003-7. PMID 9305225. http://www.jcaai.readyportal.net/file_depot/0-10000000/20000-30000/27387/folder/63948/Atopic_Derm1997.pdf.
- ↑ Nayak, Surajit; Acharjya, Basanti (2021-08-09). "Deflazacort Versus Other Glucocorticoids: A Comparison". Indian Journal of Dermatology (Ncbi.nlm.nih.gov) 53 (4): 167–170. doi:10.4103/0019-5154.44786. PMID 19882026.
- ↑ "The role of corticosteroids for pain relief in persistent pain of inflammatory arthritis: a systematic literature review". The Journal of Rheumatology. Supplement 90: 17–20. 2012. doi:10.3899/jrheum.120337. PMID 22942324.
- ↑ "Corticosteroids for the management of cancer-related pain in adults". The Cochrane Database of Systematic Reviews 2021 (4): CD010756. 2015. doi:10.1002/14651858.CD010756.pub2. PMID 25908299. PMC 8127040. https://espace.library.uq.edu.au/view/UQ:356978/UQ356978_OA.pdf.
- ↑ "Imaging and management of greater trochanteric pain syndrome". Postgraduate Medical Journal 90 (1068): 576–581. 2014. doi:10.1136/postgradmedj-2013-131828. PMID 25187570.
- ↑ 26.0 26.1 "Corticosteroid Injections Give Small and Transient Pain Relief in Rotator Cuff Tendinosis: A Meta-analysis". Clinical Orthopaedics and Related Research 475 (1): 232–243. January 2017. doi:10.1007/s11999-016-5002-1. PMID 27469590.
- ↑ 27.0 27.1 "Update on glucocorticoid action and resistance". The Journal of Allergy and Clinical Immunology 111 (1): 3–22; quiz 23. Jan 2003. doi:10.1067/mai.2003.97. PMID 12532089.
- ↑ "BCL-2 protects human and mouse Th17 cells from glucocorticoid-induced apoptosis". Allergy 71 (5): 640–650. Jan 2016. doi:10.1111/all.12840. PMID 26752231.
- ↑ "Regulation of the steady state level of Fc gamma RI mRNA by IFN-gamma and dexamethasone in human monocytes, neutrophils, and U-937 cells". Journal of Immunology 145 (1): 267–275. 1990. doi:10.4049/jimmunol.145.1.267. PMID 2141616.
- ↑ "In vivo glucocorticoid modulation of guinea pig splenic macrophage Fc gamma receptors". The Journal of Clinical Investigation 88 (1): 149–157. 1991. doi:10.1172/JCI115271. PMID 1829095.
- ↑ Werb, Zena (1980). "Hormone receptors and normal regulation of macrophage physiological function". Mononuclear phagocytes functional aspects. The Hague: M. Nijhoff. p. 825. ISBN 978-94-009-8793-7. https://books.google.com/books?id=CHXvCAAAQBAJ&q=glucocorticoid+macrophage+phagocytosis+fc+receptors&pg=PA825. "Glucocorticoids may also decrease the number of Fc receptors on macrophages, but this immunosuppressive function is controversial because of the lack of sensitivity in Fc receptor techniques and the high concentration of glucocorticoids used in previous experiments."
- ↑ "Glucocorticoids inhibit prostaglandin synthesis not only at the level of phospholipase A2 but also at the level of cyclo-oxygenase/PGE isomerase". British Journal of Pharmacology 98 (4): 1287–1295. Dec 1989. doi:10.1111/j.1476-5381.1989.tb12676.x. PMID 2514948.
- ↑ "Glucocorticoids downregulate cyclooxygenase-1 gene expression and prostacyclin synthesis in fetal pulmonary artery endothelium.". Circulation Research 84 (2): 193–200. Feb 1999. doi:10.1161/01.RES.84.2.193. PMID 9933251.
- ↑ Rang & Dale's pharmacology. Edinburgh: Churchill Livingstone. 2007. ISBN 978-0-443-06911-6. https://archive.org/details/rangdalespharmac0006dale.
- ↑ Mizokami, Tetsuya; Hamada, Katsuhiko; Maruta, Tetsushi; Higashi, Kiichiro; Tajiri, Junichi (September 2016). "Painful Radiation Thyroiditis after 131I Therapy for Graves' Hyperthyroidism: Clinical Features and Ultrasonographic Findings in Five Cases". European Thyroid Journal 5 (3): 201–206. doi:10.1159/000448398. ISSN 2235-0640. PMID 27843811.
- ↑ "The effect of prednisone and 6-methylprednisolone on mercurial diuresis in patients with refractory cardiac edema". The American Journal of the Medical Sciences 238 (5): 542–551. Nov 1959. doi:10.1097/00000441-195911000-00003. PMID 14435747.
- ↑ "Application of the newer corticosteroids to augment diuresis in congestive heart failure". The American Journal of Cardiology 1 (4): 488–496. Apr 1958. doi:10.1016/0002-9149(58)90120-6. PMID 13520608.
- ↑ "Reversal of intractable cardiac edema with prednisone". New York State Journal of Medicine 59 (4): 625–633. Feb 1959. PMID 13632954.
- ↑ "Prednisone adding to usual care treatment for refractory decompensated congestive heart failure". International Heart Journal 49 (5): 587–595. Sep 2008. doi:10.1536/ihj.49.587. PMID 18971570.
- ↑ "Potent diuretic effects of prednisone in heart failure patients with refractory diuretic resistance". The Canadian Journal of Cardiology 23 (11): 865–868. Sep 2007. doi:10.1016/s0828-282x(07)70840-1. PMID 17876376.
- ↑ "Potent potentiating diuretic effects of prednisone in congestive heart failure". Journal of Cardiovascular Pharmacology 48 (4): 173–176. Oct 2006. doi:10.1097/01.fjc.0000245242.57088.5b. PMID 17086096.
- ↑ "The glucocorticoid in acute decompensated heart failure: Dr Jekyll or Mr Hyde?". The American Journal of Emergency Medicine 30 (3): 517.e5–10. Mar 2012. doi:10.1016/j.ajem.2011.01.023. PMID 21406321.
- ↑ Henderson, Ishbel; Caiazzo, Elisabetta; McSharry, Charles; Guzik, Tomasz J.; Maffia, Pasquale (2020-10-01). "Why do some asthma patients respond poorly to glucocorticoid therapy?" (in en). Pharmacological Research 160: 105189. doi:10.1016/j.phrs.2020.105189. ISSN 1043-6618. PMID 32911071.
- ↑ "Differential effect of glucocorticoids on calcium absorption and bone mass". British Journal of Rheumatology 32 (Suppl 2): 11–14. May 1993. doi:10.1093/rheumatology/32.suppl_2.11. PMID 8495275.
- ↑ "The effect on memory of chronic prednisone treatment in patients with systemic disease". Neurology 47 (6): 1396–1402. Dec 1996. doi:10.1212/WNL.47.6.1396. PMID 8960717.
- ↑ "CORR Insights: Corticosteroid Injections Give Small and Transient Pain Relief in Rotator Cuff Tendinosis: A Meta-analysis". Clinical Orthopaedics and Related Research 475 (1): 244–246. January 2017. doi:10.1007/s11999-016-5044-4. PMID 27572298.
- ↑ "Do glucocorticoids cause spinal epidural lipomatosis? When endocrinology and spinal surgery meet". Trends in Endocrinology and Metabolism 11 (3): 86–90. Apr 2000. doi:10.1016/S1043-2760(00)00236-8. PMID 10707048.
- ↑ 48.0 48.1 "Infections associated with steroid use". Infectious Disease Clinics of North America 15 (2): 423–432, viii. Jun 2001. doi:10.1016/s0891-5520(05)70154-9. PMID 11447704.
Further reading
- "Glucocorticoids. Mood, memory, and mechanisms". Annals of the New York Academy of Sciences 1179: 19–40. Oct 2009. doi:10.1111/j.1749-6632.2009.04980.x. PMID 19906230.
External links
- Glucocorticoids at the US National Library of Medicine Medical Subject Headings (MeSH)
 |
 KSF
KSF