Gluconic acid
Topic: Chemistry
 From HandWiki - Reading time: 6 min
From HandWiki - Reading time: 6 min
| |
| Error creating thumbnail: Unable to save thumbnail to destination | |
| Names | |
|---|---|
| IUPAC name
d-Gluconic acid
| |
| Systematic IUPAC name
(2R,3S,4R,5R)-2,3,4,5,6-Pentahydroxyhexanoic acid | |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII |
|
| |
| |
| Properties | |
| C6H12O7 | |
| Molar mass | 196.155 g·mol−1 |
| Appearance | Colorless crystals |
| Density | 1.23 g/cm3[1] |
| Melting point | 131 °C (268 °F; 404 K) |
| 316 g/L[2] | |
| Acidity (pKa) | 3.86[3] |
| Hazards | |
| GHS pictograms | 
|
| GHS Signal word | Warning |
| H315, H319 | |
| PP264Script error: No such module "Preview warning".Category:GHS errors, PP264+P265Script error: No such module "Preview warning".Category:GHS errors, PP280Script error: No such module "Preview warning".Category:GHS errors, PP302+P352Script error: No such module "Preview warning".Category:GHS errors, PP305+P351+P338Script error: No such module "Preview warning".Category:GHS errors, PP321Script error: No such module "Preview warning".Category:GHS errors, PP332+P317Script error: No such module "Preview warning".Category:GHS errors, PP337+P317Script error: No such module "Preview warning".Category:GHS errors, PP362+P364Script error: No such module "Preview warning".Category:GHS errors | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
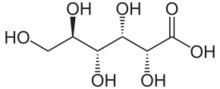
Gluconic acid is an organic compound with molecular formula C6H12O7 and condensed structural formula HOCH2(CHOH)4CO2H. A white solid, it forms the gluconate anion in neutral aqueous solution. The salts of gluconic acid are known as "gluconates". Gluconic acid, gluconate salts, and gluconate esters occur widely in nature because such species arise from the oxidation of glucose. Some drugs are injected in the form of gluconates.
Chemical structure
The chemical structure of gluconic acid consists of a six-carbon chain, with five hydroxyl groups positioned in the same way as in the open-chained form of glucose, terminating in a carboxylic acid group. It is one of the 16 stereoisomers of 2,3,4,5,6-pentahydroxyhexanoic acid.
Production
Gluconic acid is typically produced by the aerobic oxidation of glucose in the presence of the enzyme glucose oxidase. The conversion produces gluconolactone and hydrogen peroxide. The lactone spontaneously hydrolyzes to gluconic acid in water.[4]
- C
6H
12O
6 + O
2 → C
6H
10O
6 + H
2O
2 - C
6H
10O
6 + H
2O → C
6H
12O
7
Variations of glucose (or other carbohydrate-containing substrate) oxidation using fermentation.[5][6] or noble metal catalysis.[7][8]
Gluconic acid was first prepared by Hlasiwetz and Habermann in 1870[9] and involved the chemical oxidation of glucose. In 1880, Boutroux prepared and isolated gluconic acid using the glucose fermentation.[10]
Historical role in development of deep-tank fermentation
The production of gluconic acid by deep-tank fermentation (aerated, pH controlled, and stirred >1000 L tanks) of the filamentous fungi Aspergillus niger in 1929, for use as a food acidity regulator and cleaning agent, was the first successful use of deep-tank fermentation by Pfizer.[11] This expertise later led to Pfizer's successful use of deep-tank fermentation of Penicillium fungi in February 1944,[11] to rapidly scale up penicillin production, resulting in sufficient penicillin to treat the American and British battle casualties of the June 6th Allied D-Day invasion of World War II.[12]
Occurrence and uses
Gluconic acid occurs naturally in fruit, honey, and wine. As a food additive (E574[13]), it is now known as an acidity regulator.
The gluconate anion chelates Ca2+, Fe2+, K+
, Al3+, and other metals, including lanthanides and actinides. It is also used in cleaning products, where it dissolves mineral deposits, especially in alkaline solution.
Zinc gluconate injections are used to neuter male dogs.[14]
Gluconate is also used in building and construction as a concrete admixture (retarder) to slow down the cement hydration reactions, and to delay the cement setting time. It allows for a longer time to lay the concrete, or to spread the cement hydration heat over a longer period of time to avoid too high a temperature and the resulting cracking.[15][16] Retarders are mixed in to concrete when the weather temperature is high or to cast large and thick concrete slabs in successive and sufficiently well-mixed layers.
Gluconic acid aqueous solution finds application as a medium for organic synthesis.[17]
Medicine
In medicine, gluconate is used most commonly as a biologically neutral carrier of Zn2+, Ca2+, Cu2+, Fe2+, and K+
to treat electrolyte imbalance.[18]
Calcium gluconate, in the form of a gel, is used to treat burns from hydrofluoric acid;[19][20] calcium gluconate injections may be used for more severe cases to avoid necrosis of deep tissues, as well as to treat hypocalcemia in hospitalized patients. Gluconate is also an electrolyte present in certain solutions, such as "plasmalyte a", used for intravenous fluid resuscitation.[21] Quinine gluconate is a salt of gluconic acid and quinine, which is used for intramuscular injection in the treatment of malaria.
Ferrous gluconate injections have been proposed in the past to treat anemia.[22]
See also
References
- ↑ "D-Gluconic acid". https://www.ilo.org/dyn/icsc/showcard.display?p_version=2&p_card_id=1738.
- ↑ "D-Gluconic acid". https://www.acs.org/content/acs/en/molecule-of-the-week/archive/g/d-gluconic-acid.html.
- ↑ Bjerrum, J., et al. Stability Constants, Chemical Society, London, 1958.
- ↑ Wong, Chun Ming; Wong, Kwun Hei; Chen, Xiao Dong (2008). "Glucose oxidase: Natural Occurrence, Function, Properties and Industrial Applications". Applied Microbiology and Biotechnology 78 (6): 927–938. doi:10.1007/s00253-008-1407-4. PMID 18330562.
- ↑ Singh, Om V.; Kumar, Raj (2007). "Biotechnological production of gluconic acid: future implications" (in en). Applied Microbiology and Biotechnology 75 (4): 713–722. doi:10.1007/s00253-007-0851-x. ISSN 1432-0614. PMID 17525864.
- ↑ Pal, Parimal; Kumar, Ramesh; Banerjee, Subhamay (2016). "Manufacture of gluconic acid: A review towards process intensification for green production" (in en). Chemical Engineering and Processing: Process Intensification 104: 160–171. doi:10.1016/j.cep.2016.03.009. ISSN 0255-2701. Bibcode: 2016CEPPI.104..160P. https://www.sciencedirect.com/science/article/pii/S0255270116300666.
- ↑ Yan, Wenjuan; Zhang, Dongpei; Sun, Yu; Zhou, Ziqi; Du, Yihang; Du, Yiyao; Li, Yushan; Liu, Mengyuan et al. (2020). "Structural sensitivity of heterogeneous catalysts for sustainable chemical synthesis of gluconic acid from glucose" (in en). Chinese Journal of Catalysis 41 (9): 1320–1336. doi:10.1016/S1872-2067(20)63590-2. ISSN 1872-2067.
- ↑ Zhang, Qiaozhi; Wan, Zhonghao; Yu, Iris K. M.; Tsang, Daniel C. W. (2021). "Sustainable production of high-value gluconic acid and glucaric acid through oxidation of biomass-derived glucose: A critical review" (in en). Journal of Cleaner Production 312. doi:10.1016/j.jclepro.2021.127745. ISSN 0959-6526. Bibcode: 2021JCPro.31227745Z. https://www.sciencedirect.com/science/article/pii/S0959652621019636.
- ↑ Hlasiwetz, H.; Habermann, J. (1870). "Zur Kenntniss einiger Zuckerarten. (Glucose, Rohrzucker, Levulose, Sorbin, Phloroglucin.)" (in de). Berichte der Deutschen Chemischen Gesellschaft 3 (1): 486–495. doi:10.1002/cber.187000301162. ISSN 1099-0682. https://babel.hathitrust.org/cgi/pt?id=uiug.30112025693463&seq=494.
- ↑ Boutroux, L. (1880). "Sur une fermentation nouvelle du glucose" (in fr). Comptes Rendus de l'Académie des Sciences 91: 236–238. https://babel.hathitrust.org/cgi/pt?id=nyp.33433009682679&seq=244.
- ↑ 11.0 11.1 "Penicillin Production through Deep-tank Fermentation". 2008-06-12. https://www.acs.org/education/whatischemistry/landmarks/penicillin.html.
- ↑ Richards, Alfred N. (1948). ADVANCES IN MILITARY MEDICINE - Volume 1 (1st ed.). Boston: Little, Brown and Company. p. 1ii. https://digirepo.nlm.nih.gov/ext/dw/32430610RX1/PDF/32430610RX1.pdf. Retrieved 19 January 2025.
- ↑ Current EU approved additives and their E Numbers. Food Standards Agency.
- ↑ Julie K. Levy, P. Cynda Crawford, Leslie D. Appel, Emma L. Clifford (2008), Comparison of intratesticular injection of zinc gluconate versus surgical castration to sterilize male dogs. American Journal of Veterinary Research Vol. 69, No. 1, Pages 140–143. doi:10.2460/ajvr.69.1.140
- ↑ Ramachandran, V.S.; Lowery, M.S. (1992). "Conduction calorimetric investigation of the effect of retarders on the hydration of Portland cement". Thermochimica Acta 195: 373–387. doi:10.1016/0040-6031(92)80081-7. ISSN 0040-6031. Bibcode: 1992TcAc..195..373R.
- ↑ Ma, Suhua; Li, Weifeng; Zhang, Shenbiao; Ge, Dashun; Yu, Jin; Shen, Xiaodong (2015). "Influence of sodium gluconate on the performance and hydration of Portland cement". Construction and Building Materials 91: 138–144. doi:10.1016/j.conbuildmat.2015.05.068. ISSN 0950-0618.
- ↑ Lim, Han Yin; Dolzhenko, Anton V. (2021). "Gluconic acid aqueous solution: A bio-based catalytic medium for organic synthesis" (in en). Sustainable Chemistry and Pharmacy 21. doi:10.1016/j.scp.2021.100443. ISSN 2352-5541. Bibcode: 2021SusCP..2100443L. https://www.sciencedirect.com/science/article/pii/S235255412100070X.
- ↑ Mycielska, ME; Mohr, MTJ; Schmidt, K; Drexler, K; Rümmele, P; Haferkamp, S; Schlitt, HJ; Gaumann, A et al. (2019). "Potential Use of Gluconate in Cancer Therapy.". Frontiers in Oncology 9. doi:10.3389/fonc.2019.00522. PMID 31275855.
- ↑ el Saadi M. S.; Hall A. H.; Hall P. K.; Riggs B. S.; Augenstein W. L.; Rumack B. H. (1989). "Hydrofluoric acid dermal exposure". Vet Hum Toxicol 31 (3): 243–7. PMID 2741315.
- ↑ Roblin I.; Urban M.; Flicoteau D.; Martin C.; Pradeau D. (2006). "Topical treatment of experimental hydrofluoric acid skin burns by 2.5% calcium gluconate". J Burn Care Res 27 (6): 889–94. doi:10.1097/01.BCR.0000245767.54278.09. PMID 17091088.
- ↑ D. Thomas, U. Jaeger, I. Sagoschen, C. Lamberti and K. Wilhelm (2009), Intra-Arterial Calcium Gluconate Treatment After Hydrofluoric Acid Burn of the Hand. CardioVascular and Interventional Radiology, Volume 32, Number 1, pages 155–158 doi:10.1007/s00270-008-9361-1
- ↑ Paul Reznikoff and Walther F. Goebel (1937), The preparation of ferrous gluconate and its use in the treatment of hypochromic anelia in rats. Journal of Pharmacology and Experimental Therapy, volume 59 issue 2, page 182.
External links
 |
 KSF
KSF