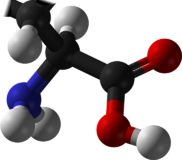
Glycine
Topic: Chemistry
 From HandWiki - Reading time: 21 min
From HandWiki - Reading time: 21 min
Glycine (symbol Gly or G;[1] /ˈɡlaɪsiːn/ (![]() listen)[2]) is an organic compound with the formula C2H5NO2, and is the simplest stable amino acid, distinguished by having a single hydrogen atom as its side chain. As one of the 20 proteinogenic amino acids, glycine is a fundamental building block of proteins in all life and is encoded by all codons starting with GG (GGU, GGC, GGA, and GGG).[3][4] Because of its minimal side chain, it is the only common amino acid that is not chiral, meaning it is superimposable on its mirror image.[5][6]
listen)[2]) is an organic compound with the formula C2H5NO2, and is the simplest stable amino acid, distinguished by having a single hydrogen atom as its side chain. As one of the 20 proteinogenic amino acids, glycine is a fundamental building block of proteins in all life and is encoded by all codons starting with GG (GGU, GGC, GGA, and GGG).[3][4] Because of its minimal side chain, it is the only common amino acid that is not chiral, meaning it is superimposable on its mirror image.[5][6]
In the body, glycine plays several crucial roles. Its small and flexible structure is vital for the formation of certain protein structures, most notably in collagen, where glycine makes up about 35% of the amino acid content and enables the tight coiling of the collagen triple helix.[3][7] Glycine disrupts the formation of alpha-helices in secondary protein structure, in favor instead of random coils.[8] Beyond its structural role, glycine functions as an inhibitory neurotransmitter in the central nervous system,[9] particularly in the spinal cord and brainstem, where it helps regulate motor and sensory signals. Disruption of glycine signaling can lead to severe neurological disorders and motor dysfunction;[10] for example, the tetanus toxin causes spastic paralysis by blocking glycine release.[11] It also serves as a key precursor for the synthesis of other important biomolecules, including the porphyrins that form heme in blood and the purines used to build DNA and RNA.[3]
Glycine is a white, sweet-tasting crystalline solid, leading to its name from Greek word glykys (Greek: γλυκύς) or "sweet".[12][2] While the body can synthesize it, it is also obtained from the diet and produced industrially by chemical synthesis for use as a food additive, a nutritional supplement, and an intermediate in the manufacture of products such as the herbicide glyphosate.[13] In aqueous solutions, glycine exists predominantly as a zwitterion (H3N+CH2COO-), a polar molecule with both a positive and negative charge, making it highly soluble in water.[14] It can also fit into hydrophobic environments due to its minimal side chain.[15]
History and etymology
Glycine was discovered in 1820 by French chemist Henri Braconnot when he hydrolyzed gelatin by boiling it with sulfuric acid.[16] He originally called it "sugar of gelatin",[17][18] but French chemist Jean-Baptiste Boussingault showed in 1838 that it contained nitrogen.[19] In 1847 American scientist Eben Norton Horsford, then a student of the German chemist Justus von Liebig, proposed the name "glycocoll";[20][21] however, the Swedish chemist Berzelius suggested the simpler current name a year later.[22][23] The name comes from the Greek word γλυκύς "sweet tasting"[24] (which is also related to the prefixes glyco- and gluco-, as in glycoprotein and glucose). In 1858, the French chemist Auguste Cahours determined that glycine was an amine of acetic acid.[25]
Production
Although glycine can be isolated from hydrolyzed proteins, this route is not used for industrial production, as it can be manufactured more conveniently by chemical synthesis.[26] The two main processes are amination of chloroacetic acid with ammonia, giving glycine and hydrochloric acid,[27] and the Strecker amino acid synthesis,[28] which is the main synthetic method in the United States and Japan.[29] About 15 thousand tonnes are produced annually in this way.[30]
Glycine is also co-generated as an impurity in the synthesis of EDTA, arising from reactions of the ammonia co-product.[31]
Chemical reactions
Its acid–base properties are most important. In aqueous solution, glycine is amphoteric: below pH = 2.4, it converts to the ammonium cation called glycinium. Above about pH 9.6, it converts to glycinate.
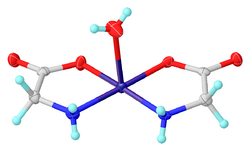
Glycine functions as a bidentate ligand for many metal ions, forming amino acid complexes.[32] A typical complex is Cu(glycinate)2, i.e. Cu(H2NCH2CO2)2, which exists both in cis and trans isomers.[33][34]
With acid chlorides, glycine converts to the amidocarboxylic acid, such as hippuric acid[35] and acetylglycine.[36] With nitrous acid, one obtains glycolic acid (van Slyke determination). With methyl iodide, the amine becomes quaternized to give trimethylglycine, a natural product:
- H3N+CH2COO− + 3 CH3I → (CH3)3N+CH2COO− + 3 HI
Glycine condenses with itself to give peptides, beginning with the formation of glycylglycine:[37]
- 2 H3N+CH2COO− → H3N+CH2CONHCH2COO− + H2O
Pyrolysis of glycine or glycylglycine gives 2,5-diketopiperazine, the cyclic diamide.[38]
Glycine forms esters with alcohols. They are often isolated as their hydrochloride, such as glycine methyl ester hydrochloride. Otherwise, the free ester tends to convert to diketopiperazine.
As a bifunctional molecule, glycine reacts with many reagents. These can be classified into N-centered and carboxylate-center reactions.
Metabolism
Biosynthesis
Glycine is not strictly essential to the human diet, as it is biosynthesized in the body. However, it is considered semi-essential in that the amount that can be biosynthesized is insufficient for all metabolic uses.[39]
It can be synthesized from the amino acid serine, which is in turn derived from 3-phosphoglycerate. In most organisms, the enzyme serine hydroxymethyltransferase catalyses this transformation via the cofactor pyridoxal phosphate:[40]
- serine + tetrahydrofolate → glycine + N5,N10-methylene tetrahydrofolate + H2O
In E. coli, antibiotics that target folate depletes the supply of active tetrahydrofolates, halting glycine biosynthesis as a consequence.[41]
In the liver of vertebrates, glycine synthesis is catalyzed by glycine synthase (also called glycine cleavage enzyme). This conversion is readily reversible:[40]
In addition to being synthesized from serine, glycine can also be derived from threonine, choline or hydroxyproline via inter-organ metabolism of the liver and kidneys.[42]
Degradation
Glycine is degraded via three pathways. The predominant pathway in animals and plants is the reverse of the glycine synthase pathway mentioned above. In this context, the enzyme system involved is usually called the glycine cleavage system:[40]
- Glycine + tetrahydrofolate + NAD+ ⇌ CO2 + NH+4 + N5,N10-methylene tetrahydrofolate + NADH + H+
In the second pathway, glycine is degraded in two steps. The first step is the reverse of glycine biosynthesis from serine with serine hydroxymethyl transferase. Serine is then converted to pyruvate by serine dehydratase.[40]
In the third pathway of its degradation, glycine is converted to glyoxylate by D-amino acid oxidase. Glyoxylate is then oxidized by hepatic lactate dehydrogenase to oxalate in an NAD+-dependent reaction.[40]
The half-life of glycine and its elimination from the body varies significantly based on dose.[43] In one study, the half-life varied between 0.5 and 4.0 hours.[43]
Physiological function
The principal function of glycine is it acts as a precursor to proteins. Most proteins incorporate only small quantities of glycine, a notable exception being collagen, which contains about 35% glycine due to its periodically repeated role in the formation of collagen's helix structure in conjunction with hydroxyproline.[40][44] In the genetic code, glycine is coded by all codons starting with GG, namely GGU, GGC, GGA and GGG.[4]
As a biosynthetic intermediate
In higher eukaryotes, δ-aminolevulinic acid, the key precursor to porphyrins, is biosynthesized from glycine and succinyl-CoA by the enzyme ALA synthase. Glycine provides the central C2N subunit of all purines.[40]
As a neurotransmitter
Glycine is an inhibitory neurotransmitter in the central nervous system, especially in the spinal cord, brainstem, and retina. When glycine receptors are activated, chloride enters the neuron via ionotropic receptors, causing an inhibitory postsynaptic potential (IPSP). Strychnine is a strong antagonist at ionotropic glycine receptors, whereas bicuculline is a weak one. Glycine is a required co-agonist along with glutamate for NMDA receptors. In contrast to the inhibitory role of glycine in the spinal cord, this behaviour is facilitated at the (NMDA) glutamatergic receptors which are excitatory.[45] The -1">50 of glycine is 7930 mg/kg in rats (oral),[46] and it usually causes death by hyperexcitability.
As a toxin conjugation agent
Glycine conjugation pathway has not been fully investigated.[47] Glycine is thought to be a hepatic detoxifier of a number endogenous and xenobiotic organic acids.[48] Bile acids are normally conjugated to glycine in order to increase their solubility in water.[49]
The human body rapidly clears sodium benzoate by combining it with glycine to form hippuric acid which is then excreted.[50] The metabolic pathway for this begins with the conversion of benzoate by butyrate-CoA ligase into an intermediate product, benzoyl-CoA,[51] which is then metabolized by glycine N-acyltransferase into hippuric acid.[52]
Uses
In the US, glycine is typically sold in two grades: United States Pharmacopeia ("USP"), and technical grade. USP grade sales account for approximately 80 to 85 percent of the U.S. market for glycine. If purity greater than the USP standard is needed, for example for intravenous injections, a more expensive pharmaceutical grade glycine can be used. Technical grade glycine, which may or may not meet USP grade standards, is sold at a lower price for use in industrial applications, e.g., as an agent in metal complexing and finishing.[53]
Animal and human foods
Glycine is not widely used in foods for its nutritional value, except in infusions. Instead, glycine's role in food chemistry is as a flavorant. It is mildly sweet, and it counters the aftertaste of saccharine. It also has preservative properties, perhaps owing to its complexation to metal ions. Metal glycinate complexes, e.g. copper(II) glycinate are used as supplements for animal feeds.[30]
As of 1971[update], the U.S. Food and Drug Administration "no longer regards glycine and its salts as generally recognized as safe for use in human food",[55] and only permits food uses of glycine under certain conditions.[56]
Glycine has been researched for its potential to extend life.[57][58] The proposed mechanisms of this effect are its ability to clear methionine from the body, and activating autophagy.[57]
Chemical feedstock
Glycine is an intermediate in the synthesis of a variety of chemical products. It is used in the manufacture of the herbicides glyphosate,[59] iprodione, glyphosine, imiprothrin, and eglinazine.[30] It is used as an intermediate of antibiotics such as thiamphenicol.[60]
Laboratory research
Glycine is a significant component of some solutions used in the SDS-PAGE method of protein analysis. It serves as a buffering agent, maintaining pH and preventing sample damage during electrophoresis.[61] Glycine is also used to remove protein-labeling antibodies from Western blot membranes to enable the probing of numerous proteins of interest from SDS-PAGE gel. This allows more data to be drawn from the same specimen, increasing the reliability of the data, reducing the amount of sample processing, and number of samples required.[62] This process is known as stripping.
Presence in space
The presence of glycine outside the Earth was confirmed in 2009, based on the analysis of samples that had been taken in 2004 by the NASA spacecraft Stardust from comet Wild 2 and subsequently returned to Earth. Glycine had previously been identified in the Murchison meteorite in 1970.[63] The discovery of glycine in outer space bolstered the hypothesis of so-called soft-panspermia, which claims that the "building blocks" of life are widespread throughout the universe.[64] In 2016, detection of glycine within Comet 67P/Churyumov–Gerasimenko by the Rosetta spacecraft was announced.[65]
The detection of glycine outside the Solar System in the interstellar medium has been debated.[66]
Evolution
Glycine is proposed to be defined by early genetic codes.[67][68][69][70] For example, low complexity regions (in proteins), that may resemble the proto-peptides of the early genetic code are highly enriched in glycine.[70]
Presence in foods
| Food | Percentage content by weight (g/100g) |
|---|---|
| Dry unsweetened gelatin powder | 19 |
| Snacks, pork skins | 11.04 |
| Sesame seeds flour (low fat) | 3.43 |
| Beverages, protein powder (soy-based) | 2.37 |
| Seeds, safflower seed meal, partially defatted | 2.22 |
| Meat, bison, beef and others (various parts) | 1.5–2.0 |
| Gelatin desserts | 1.96 |
| Seeds, pumpkin and squash seed kernels | 1.82 |
| Turkey, all classes, back, meat and skin | 1.79 |
| Chicken, broilers or fryers, meat and skin | 1.74 |
| Pork, ground, 96% lean / 4% fat, cooked, crumbles | 1.71 |
| Bacon and beef sticks | 1.64 |
| Peanuts | 1.63 |
| Crustaceans, spiny lobster | 1.59 |
| Spices, mustard seed, ground | 1.59 |
| Salami | 1.55 |
| Nuts, butternuts, dried | 1.51 |
| Fish, salmon, pink, canned, drained solids | 1.42 |
| Almonds | 1.42 |
| Fish, mackerel | 0.93 |
| Cereals ready-to-eat, granola, homemade | 0.81 |
| Leeks, (bulb and lower-leaf portion), freeze-dried | 0.7 |
| Cheese, parmesan (and others), grated | 0.56 |
| Soybeans, green, cooked, boiled, drained, without salt | 0.51 |
| Bread, protein (includes gluten) | 0.47 |
| Egg, whole, cooked, fried | 0.47 |
| Beans, white, mature seeds, cooked, boiled, with salt | 0.38 |
| Lentils, mature seeds, cooked, boiled, with salt | 0.37 |
See also
References
- ↑ "Nomenclature and Symbolism for Amino Acids and Peptides". IUPAC-IUB Joint Commission on Biochemical Nomenclature. 1983. http://www.chem.qmul.ac.uk/iupac/AminoAcid/AA1n2.html.
- ↑ 2.0 2.1 "Glycine | Definition of glycine in English by Oxford Dictionaries". https://en.oxforddictionaries.com/definition/glycine.
- ↑ 3.0 3.1 3.2 Berg, Jeremy M.; Tymoczko, John L.; Gatto, Gregory J. Jr.; Stryer, Lubert (2015). Biochemistry (8th ed.). New York: W. H. Freeman and Company. p. 35. ISBN 978-1-4641-2610-9.
- ↑ 4.0 4.1 "The Influence of the Selection at the Amino Acid Level on Synonymous Codon Usage from the Viewpoint of Alternative Genetic Codes". International Journal of Molecular Sciences 24 (2): 1185. January 2023. doi:10.3390/ijms24021185. PMID 36674703.
- ↑ "Achiral amino acid glycine acts as an origin of homochirality in asymmetric autocatalysis". Organic & Biomolecular Chemistry 17 (17): 4200–4203. April 2019. doi:10.1039/C9OB00345B. PMID 30932119.
- ↑ IUPAC, ed (2014). "achiral". Compendium of Chemical Terminology (2nd ed.). International Union of Pure and Applied Chemistry. doi:10.1351/goldbook.A00292. https://goldbook.iupac.org/terms/view/A00292.
- ↑ Nelson, David L.; Cox, Michael M. (2021). Lehninger Principles of Biochemistry (8th ed.). New York: W.H. Freeman. p. 129. ISBN 978-1-319-22800-2.
- ↑ "A helix propensity scale based on experimental studies of peptides and proteins". Biophysical Journal 75 (1): 422–427. 1998. doi:10.1016/S0006-3495(98)77529-0. PMID 9649402. Bibcode: 1998BpJ....75..422N.
- ↑ "Molecular biology of glycinergic neurotransmission". Molecular Neurobiology 14 (3): 117–142. June 1997. doi:10.1007/BF02740653. PMID 9294860.
- ↑ "Toxicology of the Neuromuscular Junction". Comprehensive Toxicology. 2018. pp. 259–282. doi:10.1016/B978-0-12-801238-3.99198-0. ISBN 978-0-08-100601-6.
- ↑ Neuroscience (6th ed.). Sunderland, Massachusetts: Sinauer Associates. 2018. pp. 132–133. ISBN 978-1-60535-380-7.
- ↑ Kihara, H.; Yamamoto, Y.; Sato, T.; Yamazaki, Y.; Sakakibara, S.; Yamaguchi, S.; Trans-yamazaki, M.; Inokuchi, K. (2004). "Kirk-Othmer Encyclopedia of Chemical Technology". Kirk-Othmer Encyclopedia of Chemical Technology. John Wiley & Sons. doi:10.1002/0471238961.0113091411090801.a01.pub2.
- ↑ Drauz, Karlheinz; Gröger, Harald; Han, Oliver (2000). "Ullmann's Encyclopedia of Industrial Chemistry". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a02_057. ISBN 3-527-30673-0.
- ↑ Haynes, William M., ed (2017). CRC Handbook of Chemistry and Physics (97th ed.). Boca Raton: CRC Press. p. 5-92. ISBN 978-1-4987-5429-3.
- ↑ "Glycine Metabolism and Its Alterations in Obesity and Metabolic Diseases". Nutrients 11 (6): 1356. June 2019. doi:10.3390/nu11061356. PMID 31208147.
- ↑ The chemical composition of the proteins. Monographs on biochemistry. Part I. Analysis (2nd ed.). London: Longmans, Green and Co.. 1912. p. 82. https://books.google.com/books?id=7JM8AAAAIAAJ&pg=PA112. Retrieved January 18, 2010.
- ↑ "Sur la conversion des matières animales en nouvelles substances par le moyen de l'acide sulfurique" (in fr). Annales de Chimie et de Physique. 2nd series 13: 113–125 [114]. 1820. https://babel.hathitrust.org/cgi/pt?id=hvd.hx3dvk;view=1up;seq=119.
- ↑ One Thousand Experiments in Chemistry: With Illustrations of Natural Phenomena; and Practical Observations on the Manufacturing and Chemical Processes at Present Pursued in the Successful Cultivation of the Useful Arts .... Sir R. Phillips and Company. 1822. p. 557. https://archive.org/details/onethousandexpe01mackgoog.
- ↑ Boussingault (1838). "Sur la composition du sucre de gélatine et de l'acide nitro-saccharique de Braconnot" (in fr). Comptes Rendus 7: 493–495. https://babel.hathitrust.org/cgi/pt?id=mdp.39015035450702;view=1up;seq=515.
- ↑ "Glycocoll (gelatine sugar) and some of its products of decomposition". The American Journal of Science and Arts. 2nd series 3: 369–381. 1847. https://babel.hathitrust.org/cgi/pt?id=hvd.32044102902764;view=1up;seq=381.
- ↑ Ihde, Aaron J. (1984). The Development of Modern Chemistry. Courier Corporation. p. 167. ISBN 978-0-486-64235-2. https://books.google.com/books?id=89BIAwAAQBAJ&pg=PA167.
- ↑ Jahres-Bericht über die Fortschritte der Chemie und Mineralogie (Annual Report on the Progress of Chemistry and Mineralogy). 47. Tübigen, (Germany): Laupp. 1848. p. 654. https://books.google.com/books?id=mDc4AQAAIAAJ&q=%22glycin%22&pg=PA654. From p. 654: "Er hat dem Leimzucker als Basis den Namen Glycocoll gegeben. ... Glycin genannt werden, und diesen Namen werde ich anwenden." (He [i.e., the American scientist Eben Norton Horsford, then a student of the German chemist Justus von Liebig] gave the name "glycocoll" to Leimzucker [sugar of gelatine], a base. This name is not euphonious and has besides the flaw that it clashes with the names of the rest of the bases. It is compounded from γλυχυς (sweet) and χολλα (animal glue). Since this organic base is the only [one] which tastes sweet, then it can much more briefly be named "glycine", and I will use this name.)
- ↑ Nye, Mary Jo (1999). Before Big Science: The Pursuit of Modern Chemistry and Physics, 1800–1940. Harvard University Press. p. 141. ISBN 978-0-674-06382-2. https://books.google.com/books?id=qKjxtZvnBKQC&pg=PA141.
- ↑ "glycine". http://oxforddictionaries.com/definition/american_english/glycine.
- ↑ "Recherches sur les acides amidés" (in fr). Comptes Rendus 46: 1044–1047. 1858. https://babel.hathitrust.org/cgi/pt?id=umn.31951d00008355e;view=1up;seq=1050.
- ↑ Okafor, Nduka (2016). Modern Industrial Microbiology and Biotechnology. CRC Press. p. 385. ISBN 978-1-4398-4323-9. https://books.google.com/books?id=PTm1CwAAQBAJ&pg=PA385.
- ↑ "Hippuric acid". Organic Syntheses 12: 40. 1932. http://www.orgsyn.org/demo.aspx?prep=cv2p0328.; Collective Volume, 2, pp. 328
- ↑ Kirk-Othmer Food and Feed Technology, 2 Volume Set. John Wiley & Sons. 2007. p. 38. ISBN 978-0-470-17448-7. https://books.google.com/books?id=f--1V1ftgtsC&pg=PA38.
- ↑ "Glycine Conference (prelim)". USITC. http://www.usitc.gov/trade_remedy/731_ad_701_cvd/investigations/2007/glycine_from_india_japan_korea/preliminary/DOC/Glycine%20Conference%20%28prelim%29.wpd.
- ↑ 30.0 30.1 30.2 Drauz, Karlheinz; Grayson, Ian; Kleemann, Axel; Krimmer, Hans-Peter; Leuchtenberger, Wolfgang; Weckbecker, Christoph (2007). "Amino Acids". Ullmann's Encyclopedia of Industrial Chemistry. doi:10.1002/14356007.a02_057.pub2. ISBN 978-3-527-30385-4.
- ↑ "Ullmann's Encyclopedia of Industrial Chemistry". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. 2005. doi:10.1002/14356007.a10_095.
- ↑ Tomiyasu, Hiroshi.; Gordon, Gilbert. (April 1976). "Ring closure in the reaction of metal chelates. Formation of the bidentate oxovanadium(IV)-glycine complex". Inorganic Chemistry 15 (4): 870–874. doi:10.1021/ic50158a027.
- ↑ "Combined Ab Initio Computational and Infrared Spectroscopic Study of the cis- and trans-Bis(glycinato)copper(II) Complexes in Aqueous Environment". The Journal of Physical Chemistry Letters 4 (9): 1502–1506. May 2013. doi:10.1021/jz400288c. PMID 26282305.
- ↑ "X-ray Absorption Study of Copper(II)−Glycinate Complexes in Aqueous Solution". The Journal of Physical Chemistry B 102 (17): 3114–3122. April 1998. doi:10.1021/jp973476m.
- ↑ "Hippuric Acid". Org. Synth. 12: 40. 1932. doi:10.15227/orgsyn.012.0040.
- ↑ "Acetylglycine". Org. Synth. 19: 4. 1939. doi:10.15227/orgsyn.019.0004.
- ↑ "Peptide bond formation via glycine condensation in the gas phase". The Journal of Physical Chemistry B 118 (29): 8583–8590. July 2014. doi:10.1021/jp504924c. PMID 24992687.
- ↑ "Insights into glycine pyrolysis mechanisms: Integrated experimental and molecular dynamics/DFT simulation studies". Fuel 351. November 2023. doi:10.1016/j.fuel.2023.128949. Bibcode: 2023Fuel..35128949L.
- ↑ Meléndez-Hevia, Enrique; De Paz-Lugo, Patricia; Cornish-Bowden, Athel; Cárdenas, María Luz (December 2009). "A weak link in metabolism: the metabolic capacity for glycine biosynthesis does not satisfy the need for collagen synthesis". Journal of Biosciences 34 (6): 853–872. doi:10.1007/s12038-009-0100-9. ISSN 0973-7138. PMID 20093739.
- ↑ 40.0 40.1 40.2 40.3 40.4 40.5 40.6 Nelson, David L.; Cox, Michael M. (2005), Principles of Biochemistry (4th ed.), New York: W. H. Freeman, pp. 127, 675–77, 844, 854, ISBN 0-7167-4339-6
- ↑ "Antifolate-induced depletion of intracellular glycine and purines inhibits thymineless death in E. coli". ACS Chemical Biology 5 (8): 787–795. August 2010. doi:10.1021/cb100096f. PMID 20553049.
- ↑ "Glycine metabolism in animals and humans: implications for nutrition and health". Amino Acids 45 (3): 463–477. September 2013. doi:10.1007/s00726-013-1493-1. PMID 23615880.
- ↑ 43.0 43.1 "Dose-dependent half-life of glycine". Urological Research 21 (4): 289–291. 1993. doi:10.1007/BF00307714. PMID 8212419.
- ↑ "Fish bone chemistry and ultrastructure: implications for taphonomy and stable isotope analysis". Journal of Archaeological Science 38 (12): 3358–3372. 2011. doi:10.1016/j.jas.2011.07.022. Bibcode: 2011JArSc..38.3358S. https://uwo.academia.edu/PaulSzpak/Papers/827788/Fish_Bone_Chemistry_and_Ultrastructure_Implications_for_Taphonomy_and_Stable_Isotope_Analysis.
- ↑ Liu, Yun; Zhang, Juntian (October 2000). "Recent development in NMDA receptors". Chinese Medical Journal 113 (10): 948–56. PMID 11775847.
- ↑ "Safety (MSDS) data for glycine". The Physical and Theoretical Chemistry Laboratory Oxford University. 2005. http://physchem.ox.ac.uk/MSDS/GL/glycine.html.
- ↑ "Conservation of the coding regions of the glycine N-acyltransferase gene further suggests that glycine conjugation is an essential detoxification pathway". Gene 571 (1): 126–134. October 2015. doi:10.1016/j.gene.2015.06.081. PMID 26149650.
- ↑ "A new perspective on the importance of glycine conjugation in the metabolism of aromatic acids". Drug Metabolism Reviews 46 (3): 343–361. August 2014. doi:10.3109/03602532.2014.908903. PMID 24754494.
- ↑ "Bile Acid Physiology". Annals of Hepatology 16 (Suppl. 1: s3–105): s4–s14. November 2017. doi:10.5604/01.3001.0010.5493. PMID 29080336.
- ↑ "Final report on the safety assessment of Benzyl Alcohol, Benzoic Acid, and Sodium Benzoate". International Journal of Toxicology 20 Suppl 3 (3_suppl): 23–50. January 2001. doi:10.1080/10915810152630729. PMID 11766131.
- ↑ "butyrate-CoA ligase". BRENDA. Technische Universität Braunschweig.. https://www.brenda-enzymes.org/php/result_flat.php4?ecno=6.2.1.2&Suchword=&organism%5B%5D=Homo+sapiens&show_tm=0. Substrate/Product
- ↑ "glycine N-acyltransferase". BRENDA. Technische Universität Braunschweig.. https://www.brenda-enzymes.org/php/result_flat.php4?ecno=2.3.1.13&Suchword=&organism%5B%5D=Homo+sapiens&show_tm=0. Substrate/Product
- ↑ "Glycine From Japan and Korea". U.S. International Trade Commission. January 2008. http://www.usitc.gov/publications/701_731/pub3980.pdf.
- ↑ "A Redetermination of cis-Aquabis(glycinato-κ2N,O)copper(II)". Acta Crystallogr. E 60 (12): m1949–m1951. 2004. doi:10.1107/S1600536804030041.
- ↑ "eCFR :: 21 CFR 170.50 – Glycine (aminoacetic acid) in food for human consumption.". https://www.ecfr.gov/current/title-21/chapter-I/subchapter-B/part-170/subpart-C/section-170.50.
- ↑ "eCFR :: 21 CFR 172.812 – Glycine". https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfCFR/CFRSearch.cfm?fr=172.812.
- ↑ 57.0 57.1 "Glycine and aging: Evidence and mechanisms". Ageing Research Reviews 87. June 2023. doi:10.1016/j.arr.2023.101922. PMID 37004845.
- ↑ "The effect of glycine administration on the characteristics of physiological systems in human adults: A systematic review". GeroScience 46 (1): 219–239. February 2024. doi:10.1007/s11357-023-00970-8. PMID 37851316.
- ↑ Stahl, Shannon S.; Alsters, Paul L. (2016). Liquid Phase Aerobic Oxidation Catalysis: Industrial Applications and Academic Perspectives. John Wiley & Sons. p. 268. ISBN 978-3-527-69015-2. https://books.google.com/books?id=z5-tDAAAQBAJ&pg=PA268.
- ↑ "An Improved Industrial Synthesis of Florfenicol plus an Enantioselective Total Synthesis of Thiamphenicol and Florfenicol". The Journal of Organic Chemistry (American Chemical Society) 62 (9): 2996–2998. 1997-05-02. doi:10.1021/jo961479+. PMID 11671665. https://pubs.acs.org/doi/10.1021/jo961479%2B. Retrieved 2025-05-31.
- ↑ "Tricine-SDS-PAGE". Nature Protocols 1 (1): 16–22. 2006-05-12. doi:10.1038/nprot.2006.4. PMID 17406207.
- ↑ "Multiple immunoreplica Technique: screening for specific proteins with a series of different antibodies using one polyacrylamide gel". Analytical Biochemistry 111 (2): 385–392. March 1981. doi:10.1016/0003-2697(81)90577-7. PMID 6166216.
- ↑ "Evidence for extraterrestrial amino-acids and hydrocarbons in the Murchison meteorite". Nature 228 (5275): 923–926. December 1970. doi:10.1038/228923a0. PMID 5482102. Bibcode: 1970Natur.228..923K.
- ↑ "Building block of life found on comet". Thomson Reuters 2009. 18 August 2009. https://www.reuters.com/article/scienceNews/idUSTRE57H02I20090818.
- ↑ European Space Agency (27 May 2016). "Rosetta's comet contains ingredients for life". http://sci.esa.int/rosetta/57858-rosettas-comet-contains-ingredients-for-life/.
- ↑ "Reversal operator to compensate polarization random drifts in quantum communications". Optics Express 28 (4): 5035–5049. February 2020. doi:10.1086/426677. PMID 32121732. Bibcode: 2005ApJ...619..914S.
- ↑ "Consensus temporal order of amino acids and evolution of the triplet code". Gene 261 (1): 139–151. December 2000. doi:10.1016/S0378-1119(00)00476-5. PMID 11164045.
- ↑ "A thermodynamic basis for prebiotic amino acid synthesis and the nature of the first genetic code". Astrobiology 9 (5): 483–490. June 2009. doi:10.1089/ast.2008.0280. PMID 19566427. Bibcode: 2009AsBio...9..483H.
- ↑ "The complex evolutionary history of aminoacyl-tRNA synthetases". Nucleic Acids Research 45 (3): 1059–1068. February 2017. doi:10.1093/nar/gkw1182. PMID 28180287.
- ↑ 70.0 70.1 "Low complexity regions in the proteins of prokaryotes perform important functional roles and are highly conserved". Nucleic Acids Research 47 (19): 9998–10009. November 2019. doi:10.1093/nar/gkz730. PMID 31504783.
- ↑ "FoodData Central Search Results for 'Glycine (g)'". http://fdc.nal.usda.gov/food-search/?component=1225.
Further reading
- "Determination of refractive index and layer thickness of nm-thin films via ellipsometry". Optics Express 25 (22): 27077–27085. October 2017. doi:10.1086/375637. PMID 29092189. Bibcode: 2003ApJ...593..848K.
- Nowak, Rachel (18 July 2002). "Amino acid found in deep space". New Scientist. https://www.newscientist.com/article/dn2558-amino-acid-found-in-deep-space/.
- "A New Class of Antipsychotic Drugs: Enhancing Neurotransmission Mediated by NMDA Receptors". Psychiatric Times 25 (14). 1 December 2008. http://www.psychiatrictimes.com/display/article/10168/1357569. Retrieved 23 January 2009.
- "Organic Molecule, Amino Acid-Like, Found In Constellation Sagittarius". ScienceDaily (Press release). Max-Planck-Gesellschaft. 27 March 2008.
External links
- Glycine MS Spectrum
- Glycine
- Glycine cleavage system
- Glycine Therapy – A New Direction for Schizophrenia Treatment?
- ChemSub Online (Glycine).
- NASA scientists have discovered glycine, a fundamental building block of life, in samples of comet Wild 2 returned by NASA's Stardust spacecraft.
 |
 KSF
KSF