Gold(I,III) chloride
Topic: Chemistry
 From HandWiki - Reading time: 4 min
From HandWiki - Reading time: 4 min
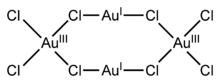
| |
| |
| Names | |
|---|---|
| Systematic IUPAC name
tetra-μ-chlorotetrachlorotetragold | |
| Other names
Mixed gold chloride, Tetragold octachloride
| |
| Identifiers | |
3D model (JSmol)
|
|
PubChem CID
|
|
| |
| |
| Properties | |
| Au4Cl8 | |
| Molar mass | 1071.490 g mol−1 |
| Appearance | black crystals |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Gold(I,III) chloride is a black solid with the chemical formula Au4Cl8. It is an example of a mixed valence compound as it contains gold in two different oxidation states; square-planar gold(III) and almost linear gold(I). The compound is photosensitive as well as air- and moisture-sensitive.
Synthesis
Gold(I,III) chloride may be prepared by the reaction of gold(III) chloride with gold carbonyl chloride[1] or carbon monoxide[2] at room temperature in thionyl chloride.
- Au2(CO)Cl4 + Au2Cl6 → COCl2 + Au4Cl8
- 2 Au2Cl6 + 2 CO → Au4Cl8 + 2 COCl2
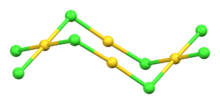
Structure and properties
Single crystals of gold(I,III) chloride are triclinic with a P1 space group and consist of discrete Au4Cl8 molecules with idealised C2h symmetry.[1] Within this the Au(I) centers are linearly coordinated with a Cl-Au-Cl bond angle of 175.0° (close to the ideal value of 180°) and an average bond length of 2.30 Å. The Au(III) centers adopt a slightly irregular square-planar conformation with the Au-Cl bond lengths for bridging chlorides (2.33 Å) being slightly longer than those of terminal chlorides (2.24 Å).
References
- ↑ 1.0 1.1 Dell'Amico, Daniela Belli; Calderazzo, Fausto; Marchetti, Fabio; Merlino, Stefano; Perego, Giovanni (1977). "X-Ray crystal and molecular structure of Au4Cl8, the product of the reduction of Au2Cl6 by Au(CO)Cl". Journal of the Chemical Society, Chemical Communications (1): 31. doi:10.1039/C39770000031.
- ↑ Dell'Amico, Daniela Belli; Calderazzo, Fausto; Marchetti, Fabio; Merlino, Stefano (1982). "Synthesis and molecular structure of [Au4Cl8], and the isolation of [Pt(CO)Cl5]− in thionyl chloride". Journal of the Chemical Society, Dalton Transactions (11): 2257. doi:10.1039/DT9820002257.
 |
 KSF
KSF